About
Signals generated by the engagement of the T cell antigen receptor are decoded and integrated by molecular complexes that form at the immunological synapse upon T cell receptor engagement. Assembly of these complexes, as well as their spatial and temporal dynamics, are finely regulated and tightly control T cell activation outcomes. A series of phosphorylation events involving both protein tyrosine kinases and serine/threonine kinases participates to this regulation. Among these signaling complexes, the one formed by the adapter proteins SLP76, GADS and LAT plays a critical role. GADS constitutively interacts with SLP76 and binds to phosphorylated LAT upon T cell receptor engagement. The LAT-GADS-SLP76 complex scaffolds the interactions of an array of signaling effector proteins, thus orchestrating T cell receptor signaling amplification (Review: Acuto et al., 2008). We investigate the molecular mechanisms underlying positive and negative regulation of these molecular complexes and how these mechanisms eventually modulate T cell responses.
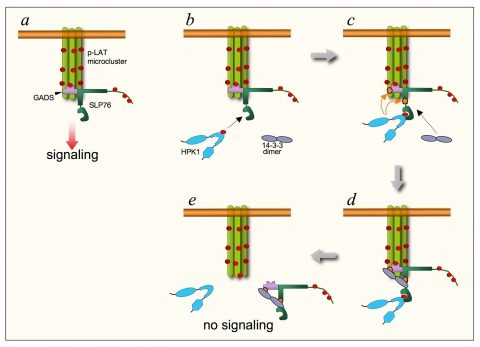
In this line, we have investigated the role of the serine-threonine kinase HPK1 (Hematopoietic Progenitor Kinase 1), that negatively regulates the stability of the T cell signalosome by phosphorylating the signaling adapters SLP76 and GADS (see figure below; Di Bartolo et al, 2007, Lasserre et al, 2011). We are currently addressing how this regulatory mechanism affects physiological and pathological T cell responses.