About
Transient viral exposure drives functionally-coordinated humoral immune responses in HIV-1 post-treatment controllers
HIV-1 post-treatment controllers (PTC) are rare individuals controlling HIV-1 infection for years after antiretroviral therapy interruption. Identification of immune correlates of control in post-treatment controllers could aid in designing effective HIV-1 vaccine and remission strategies. We performed a comprehensive immunoprofiling of the humoral response to HIV-1 in PTC. Global multivariate analyses combining clinico-virological and humoral immune data revealed very distinct profiles in PTC experiencing transient viremic episodes off therapy compared to those stably aviremic. Virally-exposed PTC displayed stronger HIV-1 humoral responses, and develop more frequently Env-specific memory B cells and cross-neutralizing antibodies (Fig.1). Both were linked to short viremic exposures, which were also accompanied by an increase in blood atypical memory B cells and activated subsets of circulating follicular helper T cells. Thus, PTC form a heterogeneous group with two distinct viral behaviours and associated immune signatures. PTC stably aviremic present “silent” humoral profiles, while those virally-exposed develop functionally robust HIV-specific B-cell and antibody responses, which may participate in controlling infection.ological level with more than hundred clinico-virological and humoral parameters being measured. | Molinos-Albert et al. Nat commun 2022 |.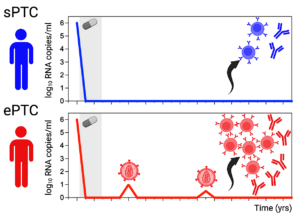
Figure 1. sPTC vs ePTC. Stably aviremic PTC (sPTC) are characterized by a low-magnitude antibody response akin to early-treated individuals on ART. In contrast, PTC experiencing transient viremic episodes (ePTC) develop a multicomponent humoral immunity against HIV-1 associating the preservation of functional cTfh-cell sub-populations, the expansion of blood Env-specific memory B cells and the production of IgG antibodies with cross-neutralizing and Fc-dependent effector activities.
Anti-V1/V3-glycan broadly HIV-1 neutralizing antibodies in a post-treatment controller
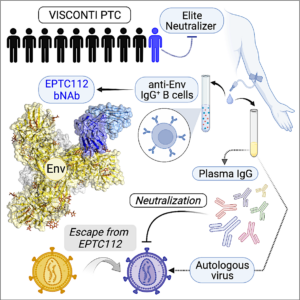
HIV-1 broadly neutralizing antibodies (bNAbs) can decrease viremia in humans, but are usually unable to counteract autologous viruses naturally escaping antibody pressure. Still, they may contribute to the natural HIV-1 control in rare individuals off antiretroviral therapy (ART). Here, we describe a novel bNAb B-cell lineage elicited in a post-treatment controller (PTC) with broad seroneutralization, EPTC112, targeting a quaternary epitope in the glycan-V3 loop supersite. The cryo-EM structure of EPTC112-BG505 SOSIP.664 complex revealed main interactions with N301 and N156 N-glycans, and the 324GDIR327 V3 loop motif. While the sole contemporaneous circulating virus identified in this PTC was resistant to EPTC112 via escape alterations in the bNAb epitope, it was potently neutralized by autologous plasma IgG antibodies. Our findings provide insights on how cross-neutralizing antibodies alter the course of HIV-1 infection in some virally-exposed PTC and may help controlling viremia off-ART, supporting their key role in designing functional HIV-1 cure strategies. | Molinos-Albert et al. Cell Host Microbe 2023 |.