About
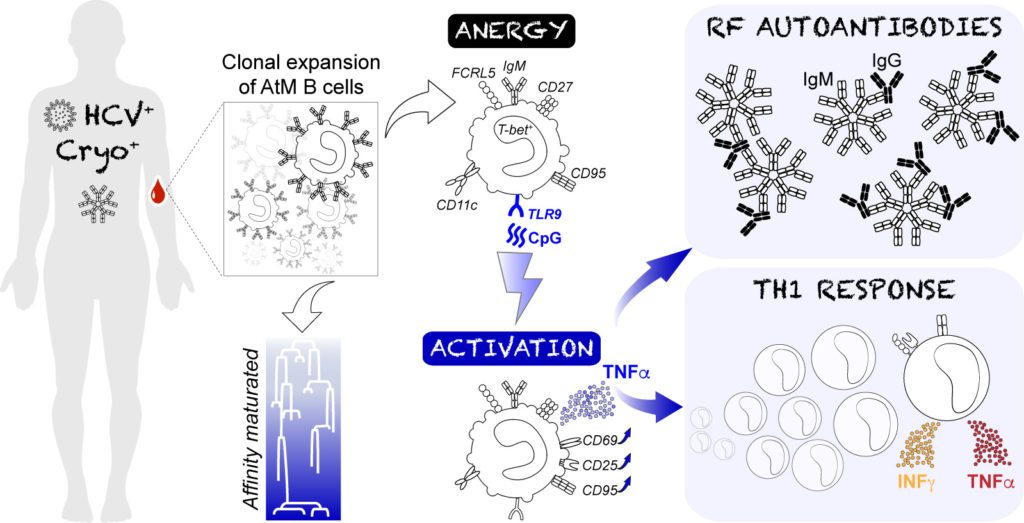
Hepatitis C virus (HCV) infection can trigger the development of an autoimmune disorder called cryobulininemic vasculatitis. HCV-associated cryobulininemic vasculatitis (HCV-CV) is mediated by mono-/oligo-clonal IgM cryoglobulins with rheumatoid factor activity, thought to be produced by atypical CD21–CD27+ memory (AtM) B cells.
In collaboration with Pr David Saadoun (La Pitié-Salpétrière Hospital, INSERM UMR_S 959), we investigated the cellular and molecular processes by which abnormally expanded AtM could participate to HCV-associated autoimmunity. We demonstrated that TLR9 stimulation in HCV-CV AtM B cells induces: (i) the secretion by AtM of TNFα and IgMs with rheumatoid factor activity; (ii) an AtM-specific transcriptional signature centered on TNFα overexpression; (iii) the stimulation and secretion of IFNγ and TNFα by effector T cells. Our direct contribution to this work has been to also show that: (i) AtM B-cell expansions display intraclonal diversity of mutated IgM with features of antigen-driven maturation; (ii) AtM antibodies do not react against ubiquitous autoantigens or HCV antigens but rather, possess rheumatoid factor (RF) activity with unique targeted epitopes on the human IgG Fc region.
Thus, our data suggest a central role of memory B cells in the defective tolerance characterizing HCV-CV patients, via the TLR9-induced reactivation of anergic AtM, which eventually mediate RF IgM autoantibody and pro-inflammatory Th1 responses.
| Comarmond*, Lorin*, Marques* et al J Hepatol 2019 |.