Track record – highlights:
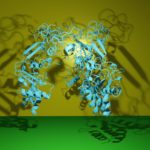
Structure determination of the Epstein-Barr virus fusogen gB. This work was done during my postdoctoral training with Theodore Jardetzky and Richard Longnecker at the Northwestern University in the USA. I conducted functional and biophysical investigations to find ways to obtain soluble and homogeneous preparations of recombinant EBV gB ectodomains, which were needed for crystallizations. I found out that the gB aggregation was driven by exposed hydrophobic sequences gB called fusion loops. These regions normally interact with target membranes and are important for membrane fusion, which I demonstrated in functional assays. By swapping the EBV fusion loop residues for the non-hydrophobic counterparts from HSV-1 gB I managed to produce soluble EBV gB ectodomains and to solve the structure by X-ray crystallography. This strategy has since been employed by others for production of soluble versions of gBs with hydrophobic fusion loops. Reference: Backovic M, Longnecker R, Jardetzky TS. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc Natl Acad Sci U S A. 2009;106(8):2880-5
Development of a system for large scale expression of recombinant Fab molecules: I established a method for expression of large quantities of recombinant Fab molecules in Drosophila S2 cells (reference 2). Fab molecules are widely used as tools to facilitate crystallization of flexible, glycosylated proteins and in cryo-EM to increase sample size and alleviate the preferential orientation problems. The S2 cells system we set up in the lab can be used for production of proteins in general as we described. Reference: Backovic M, Krey T. Stable Drosophila Cell Lines: An Alternative Approach to Exogenous Protein Expression. Methods Mol Biol. 2016;1350:349-58
Functional and structural analyses of a-herpesvirus gH/gL complex: When I joined the Structural Virology Unit directed by Félix Rey at the Institut Pasteur in Paris, I continued studying herpesviruses in collaboration with Thomas Mettenleiter at the Friedrich-Loeffler-Institut in Reims, Germany. My focused moved from the g-herpesvirus EBV to Pseudorabies virus (PrV) which belongs to the a-subfamily. I solved the structure of the PrV glycoprotein H, which was a foundation for several structure-driven mutagenesis analyses performed in collaboration with the Mettenleiter team. Reference: Backovic M, DuBois RM, Cockburn JJ, Sharff AJ, Vaney M-C, Granzow H, Klupp BG, Bricogne G, Mettenleiter TC, Rey FA. Structure of a core fragment of glycoprotein H from pseudorabies virus in complex with antibody. Proc Natl Acad Sci U S A. 2010;107(52):22635-40.
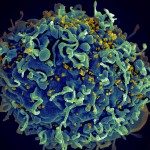
Integrin production and structural studies: I also studied glycoproteins involved in entry of CMV, a b-herpesvirus with high medical relevance. My goal was to determine the structure of CMV gH/gL bound to integrin receptor by cryo-EM in collaboration with David Veesler at the Univ. of Washington in Seattle, USA. To our disappointment the complex was not tractable by crystallography due to inherent flexibility of integrins and gH/gL, nor by cryo-EM due to low affinity binding. But we took interest in the anti-integrin Fab molecule called LM609, which we originally used to stabilize the gH/gL-integrin complex, and that was in clinical trials as a therapeutic agent. We solved the structure of the integrin-Fab complex by cryo-EM, gaining insights into the steric blocking mechanism that the Fab uses to block the integrin function. Reference: Borst AJ, James ZM, Zagotta WN, Ginsberg M, Rey FA, DiMaio F, Backovic M, Veesler D. The Therapeutic Antibody LM609 Selectively Inhibits Ligand Binding to Human αVβ3 Integrin via Steric Hindrance. Structure. 2017;25(11):1732-9.
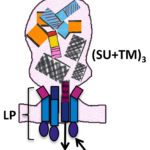
Identification of molecular determinants dictating gB interactions with membranes: Fusion loops of viral fusion proteins are typically highly conserved sequences rich in aromatic and hydrophobic residues, conducive to insertion into lipid bilayers. I was interested in why herpesvirus gB fusion loops are not conserved i.e. are hydrophobic in EBV, CMV for example, while hydrophilic in a-herpesviruses. To address this question, I used gB from PrV, which can be produced as a soluble recombinant WT protein because its fusion loops are not hydrophobic and do not need to be mutated to obtain soluble protein. I solved the structure of PrV gB ectodomains by X-ray crystallography, and designed the biochemical and functional studies, the latter performed in the Mettenleiter team. We succeeded in understanding how even the hydrophilic fusion loops of PrV gB can interact with lipids in membranes and generated a membrane insertion model that can be applied to other herpesviruses. Reference: Vallbracht M, Brun D, Tassinari M, Vaney MC, Pehau-Arnaudet G, Guardado-Calvo P, Haouz A, Klupp BG, Mettenleiter TC, Rey FA, Backovic M. Structure-Function Dissection of Pseudorabies Virus Glycoprotein B Fusion Loops. J Virol. 2018;92(1)
Discovery of structural mimicry between KSHV gH/gL and EphA2 ligands: I pursued my interest in how herpesviruses engage cellular receptors to enter cells by initiating the project on gH/gL from KSHV that binds with high affinity to EphA2 receptor, as identified by A. Hahn who is a PI in this proposal. This complex was very challenging for crystallization. I spent a considerable amount of time and gained experience in cryo-EM by collecting data on the gH/gL-EphA2 complexes for structure determination by cryo-EM single-particle analyses. Because of the elongated form of the complex, that lacked distinguishable features and I was not able to obtain useful structural data, but I did finally succeed in obtaining high-resolution data of the complex by X-ray crystallography. The structure revealed that the region in gH/gL that interacts with EphA2 adopts a fold and presents residues for interactions that resemble how EphA2 physiological ligands called ephrins bind to EphA2. To further understand if there is a functional mimicry with EphA2 I approached and established a collaboration with Kalina Hristova (John Hopkins University, Baltimore, USA), who is a leading expert in studying EphA2 signaling and interactions within cellular membranes. We showed that KSHV gH/gL, akin to ephrins, activates EphA2 signaling pathways via induction of distinct EphA2 dimers. This finding has implications for EphA2 proliferative signaling that may contribute to KSHV-related oncogenesis. I contacted Alexander Hahn while I was writing that manuscript, and this proposal is a result of our collaborative efforts and common interests in KSHV biology. Reference: Light TP, Brun D, Guardado-Calvo P, Pederzoli R, Haouz A, Neipel F, Rey FA, Hristova K, Backovic M. Human herpesvirus 8 molecular mimicry of ephrin ligands facilitates cell entry and triggers EphA2 signaling. PLoS Biol. 2021;19(9):e3001392.
For the full list of publications please check here.