Link to Pubmed [PMID] – 34124789
Link to DOI – 10.15252/embj.2020107176
EMBO J 2021 Jun; (): e107176
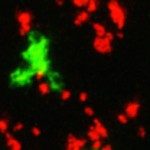
Dendritic cell (DC) activation by viral RNA sensors such as TLR3 and MDA-5 is critical for initiating antiviral immunity. Optimal DC activation is promoted by type I interferon (IFN) signaling which is believed to occur in either autocrine or paracrine fashion. Here, we show that neither autocrine nor paracrine type I IFN signaling can fully account for DC activation by poly(I:C) in vitro and in vivo. By controlling the density of type I IFN-producing cells in vivo, we establish that instead a quorum of type I IFN-producing cells is required for optimal DC activation and that this process proceeds at the level of an entire lymph node. This collective behavior, governed by type I IFN diffusion, is favored by the requirement for prolonged cytokine exposure to achieve DC activation. Furthermore, collective DC activation was found essential for the development of innate and adaptive immunity in lymph nodes. Our results establish how collective rather than cell-autonomous processes can govern the initiation of immune responses.