New J Phys 15:125026
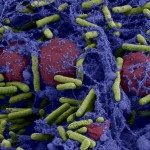
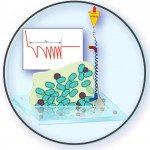
Bacteria living on surfaces form heterogeneous three-dimensional consortia known as biofilms, where they exhibit many specific properties one of which is an increased tolerance to antibiotics. Biofilms are maintained by a polymeric network and display physical properties similar to that of complex fluids. In this work, we address the question of the impact of antibiotic treatment on the physical properties of biofilms based on recently developed tools enabling the in situ mapping of biofilm local mechanical properties at the micron scale. This approach takes into account the material heterogeneity and reveals the spatial distribution of all the small changes that may occur in the structure. With an Escherichia coli biofilm, we demonstrate using in situ fluorescent labeling that the two antibiotics ofloxacin and ticarcillin—targeting DNA replication and membrane assembly, respectively—induced no detectable alteration of the biofilm mechanical properties while they killed the vast majority of the cells. In parallel, we show that a proteolytic enzyme that cleaves extracellular proteins into short peptides, but does not alter bacterial viability in the biofilm, clearly affects the mechanical properties of the biofilm structure, inducing a significant increase of the material compliance. We conclude that conventional biofilm control strategy relying on the use of biocides targeting cells is missing a key target since biofilm structural integrity is preserved. This is expected to efficiently promote biofilm resilience, especially in the presence of persister cells. In contrast, the targeting of polymer network cross-links—among which extracellular proteins emerge as major players—offers a promising route for the development of rational multi-target strategies to fight against biofilms.