Lien vers Pubmed [PMID] – 18773117
Lien DOI – e100014610.1371/journal.ppat.1000146
PLoS Pathog 2008 Sep; 4(9): e1000146
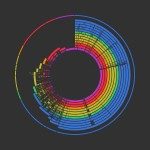
Listeria monocytogenes is a model organism for cellular microbiology and host-pathogen interaction studies and an important food-borne pathogen widespread in the environment, thus representing an attractive model to study the evolution of virulence. The phylogenetic structure of L. monocytogenes was determined by sequencing internal portions of seven housekeeping genes (3,288 nucleotides) in 360 representative isolates. Fifty-eight of the 126 disclosed sequence types were grouped into seven well-demarcated clonal complexes (clones) that comprised almost 75% of clinical isolates. Each clone had a unique or dominant serotype (4b for clones 1, 2 and 4, 1/2b for clones 3 and 5, 1/2a for clone 7, and 1/2c for clone 9), with no association of clones with clinical forms of human listeriosis. Homologous recombination was extremely limited (r/m<1 for nucleotides), implying long-term genetic stability of multilocus genotypes over time. Bayesian analysis based on 438 SNPs recovered the three previously defined lineages, plus one unclassified isolate of mixed ancestry. The phylogenetic distribution of serotypes indicated that serotype 4b evolved once from 1/2b, the likely ancestral serotype of lineage I. Serotype 1/2c derived once from 1/2a, with reference strain EGDe (1/2a) likely representing an intermediate evolutionary state. In contrast to housekeeping genes, the virulence factor internalin (InlA) evolved by localized recombination resulting in a mosaic pattern, with convergent evolution indicative of natural selection towards a truncation of InlA protein. This work provides a reference evolutionary framework for future studies on L. monocytogenes epidemiology, ecology, and virulence.