Présentation
The CyaA toxin is a 1706 residues-long bifunctional protein (Figure 1): the calmodulin-activated, catalytic domain is located in the 400 amino-proximal residues, whereas the carboxy-terminal 1306 residues are responsible for the binding of the toxin to the target cells and the translocation of the catalytic domain across the cytoplasmic membrane of these cells (Ladant et Ullmann, 1999). The CyaA toxin possesses the unique property to translocate its catalytic domain directly across the plasma membrane of targeted eukaryotic cells (Veneziano et al., 2013). Our main aim is to characterize the molecular basis of this original intoxication process. From a fundamental point of view, our objective is to gain better insights into the translocation process, that is, how the toxin partitions from solution to lipid bilayer and inserts into the membrane, and how the catalytic domain crosses the hydrophobic barrier of the membrane and refolds into a functional enzyme once in the cytosol.
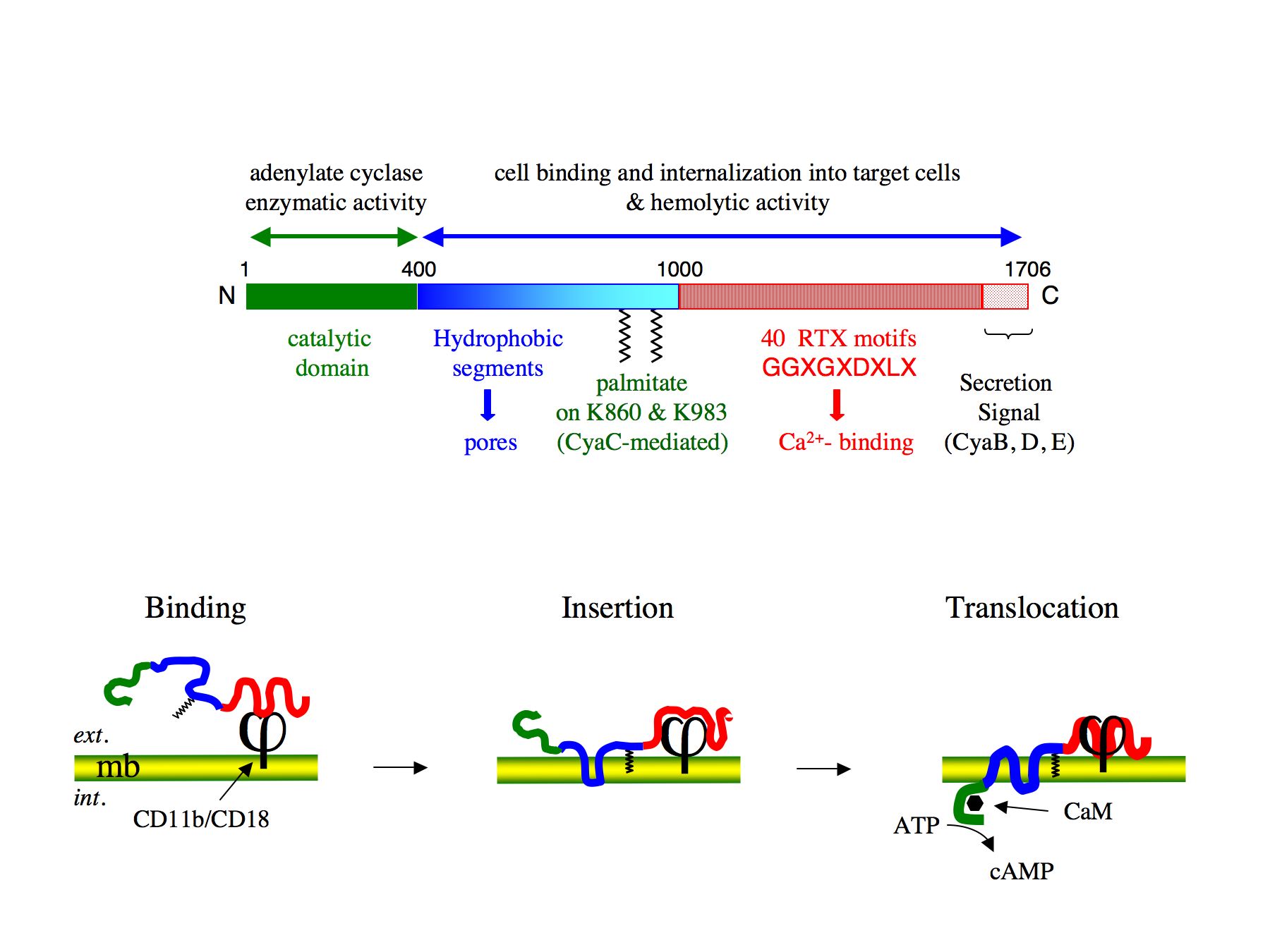
Figure 1 : Schematic model for CyaA toxin entry into target cells
A 3 stage model for CyaA invasion of target cells : (i) binding of CyaA to CD11b/CD18 receptor at the target cell surface ; (ii) insertion of hydrophobic segments of CyaA into the plasma membrane of target cells ; (iii) translocation of CyaA catalytic domain across the plasma membrane of target cells. In the cytosol CyaA interacts with calmodulin (CaM) that stimulates its catalytic activity to produce supra-physiological amount of cAMP.
Our recent studies have been focused on the biophysical characterization of different sub-domains of the CyaA toxin, by using complementary physicochemical approaches (circular dichroism, fluorescence, analytical ultracentrifugation, size-exclusion chromatography, etc).
We analyzed the conformational transitions of the N-terminal catalytic domain (AC, encompassing residues 1-384 of CyaA) induced by calmodulin-binding with the aim of delineating the molecular mechanisms of activation of the enzymatic activity by this regulatory protein, and identified specific residues that are crucial for the allosteric activation of AC by calmodulin (Karst, et al;, 2010; Selwa et al , 2014).
We further characterized a region of the CyaA polypeptide (residues 384 to 489, downstream to the AC catalytic domain) that facilitates the binding of the AC domain to lipid membrane. This region appears to directly contribute to the acquisition by the AC domain of a conformation competent for insertion into the lipid bilayer, and possibly for its translocation through the membrane (Karst, et al. 2012; Subrini et al 2013).
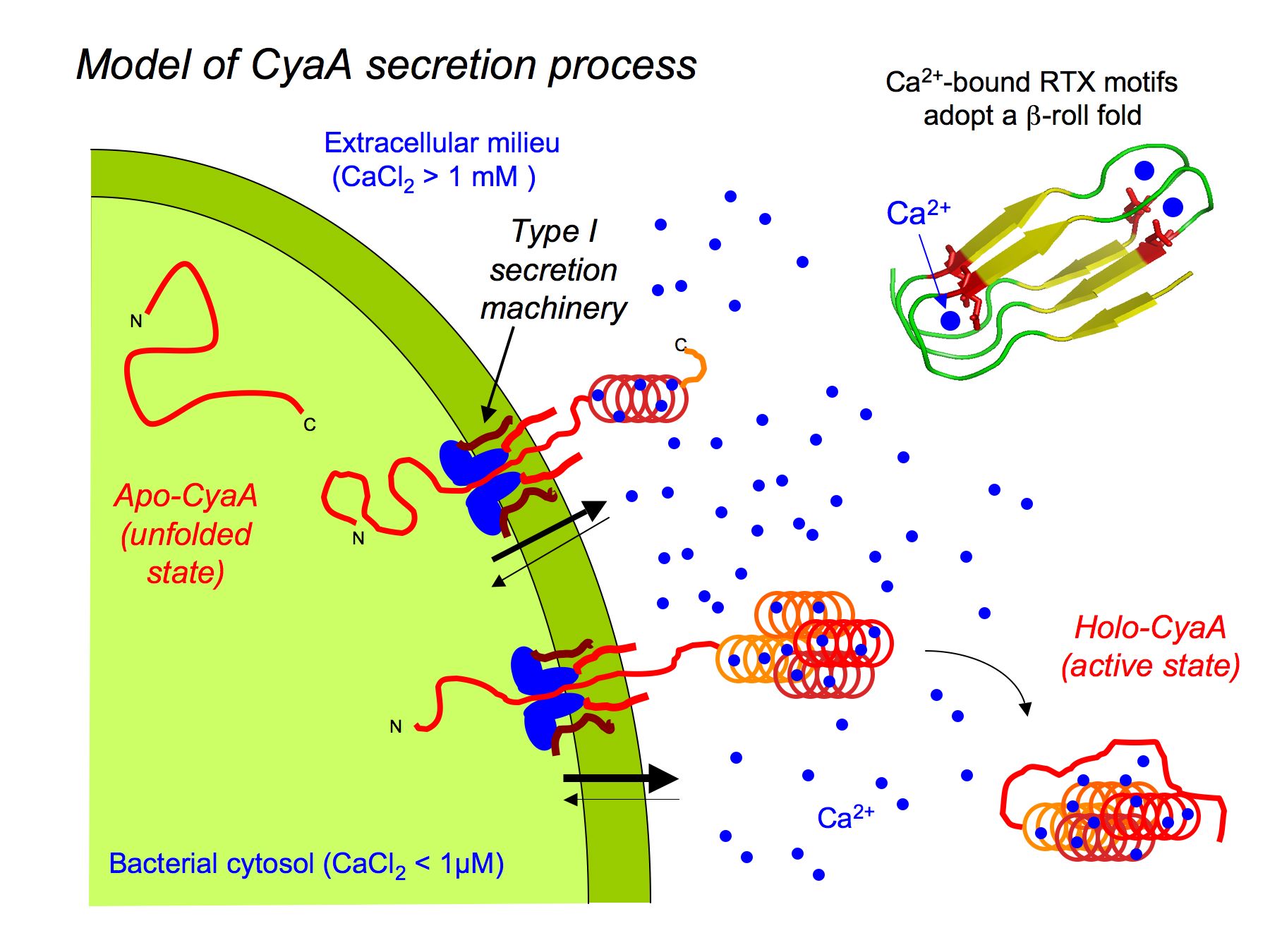
We also explored the physico-chemical properties of the receptor-binding domain (RD) of the toxin (residues 1006 to 1706), that mediates CyaA binding to the cellular receptor, the CD11b/CD18 integrin. RD contains about 40 calcium-binding nonapeptide sequences known as « Repeat in ToXin » (RTX) motifs. RD is natively disordered in the absence of calcium, and acquired stable secondary and tertiary structures upon binding of calcium (Chenal et al., 2009; Chenal et al. 2010; Sotomayor-Perez et al. 2013). The intrinsically disordered state of the RTX proteins in the apo-form could facilitate their secretion through a dedicated type I secretion system (T1SS) into the bacterial extracellular medium, where upon binding calcium ions they can fold into their active conformation (Figure 2). We propose that the calcium-induced disorder-to-order transition may be a key property of other RTX-containing proteins that are virulence factors produced by a wide variety of bacterial species. Besides, our work suggests that RD is remarkable model to explore the biophysical principles underlying the ligand-induced disorder-to-order transition in proteins.
More information on the project
Figure 2. Model for the secretion of CyaA through the type 1 secretion system (T1SS). On the upper right is a schematic model of the b-roll fold adopted by RTX motifs upon binding of Ca2+.