We are actively looking for a motivated post-doc (positions starting from Nov 2022) with experience in developmental biology and working with early mouse embryos, who is interested in extending his/her expertise to chromatin biology. Candidates will leverage state of the art cell culture models, including blastoids and 2C-like cells, tailored conditional knockout strategies developed by the group, and will couple embryology, single cell transcriptional profiling and chromatin studies to shed light on the molecular mechanisms of action of a neglected class of developmental regulators, orphan nuclear receptors, during zygotic genome activation (ZGA), early lineage segregation, and blastocyst specification. We are a motivated, young, and dynamic team operating as part of an established unit at a leading European research organisation, the Institut Pasteur in Paris. Please send candidatures to nicola.festuccia@pasteur.fr.
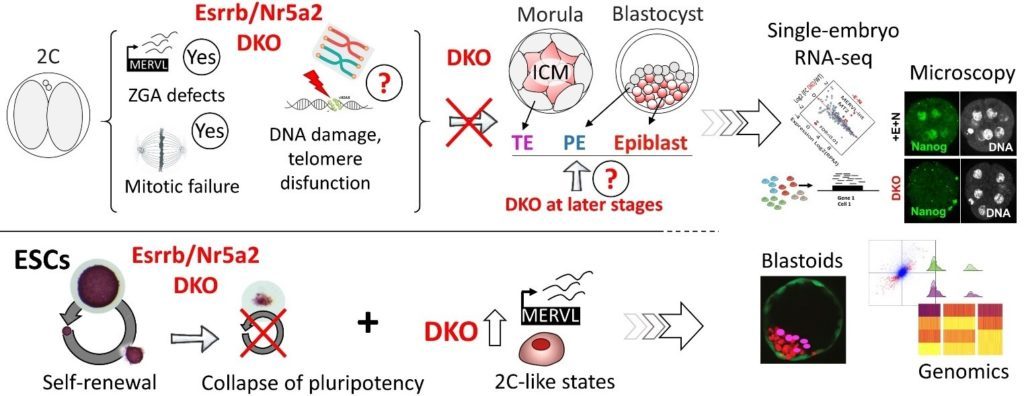
In some detail: We are interested understanding the mechanisms by which orphan nuclear receptors control the full span of developmental events that link fertilisation to the specification of pluripotency in the epiblast of pre-implantation embryos, endowing this population of cells with the ability to give rise to all tissue constituting an adult body. We have recently accumulated evidence that two such TFs, Esrrb and Nr5a2, play a decisive function from the very first days of development. The complete loss of zygotic expression of Nr5a2/Esrrb compromises the activation of many genes during the period of ZGA, but also results in a protracted expression of 2-cell specific genes in later embryos, including that of endogenous retroviral elements. Defects accumulate, leading to the appearance of overt mitotic defects around the 8-cell stage, and eventually trigger a full developmental arrest in early morulae. In this context, Nr5a2/Esrrb might preserve genome stability by specifically controlling telomere maintenance. The incipient expression of primitive endoderm and trophectoderm markers is also affected, and mutant embryos entirely fail to upregulate key pluripotency TFs. This is in line with our recent report that the conjunct activity of Esrrb and Nr5a2 is strictly required for the maintenance of pluripotency in mouse embryonic stem cells (ESCs). Taken together, our data suggest that the raising expression of Nr5a2/Esrrb likely contributes to, and drive the embryo past, the stage of ZGA, setting the stage for the cell fate choices that lead to the segregation of embryonic and extra-embryonic lineages. Based on our observations in ESCs, the two TFs may then be essential to promote the attainment of pluripotency in the epiblast. Candidates who will join our team will dissect in detail the molecular mechanisms at the base of these pivotal developmental functions.