Link to Pubmed [PMID] – 41420107
Link to DOI – 10.1038/s44321-025-00358-5
EMBO Mol Med 2025 Dec; ():
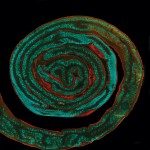
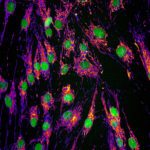
Mutations in CHCHD10, a mitochondrial intermembrane space (IMS) protein implicated in proteostasis and cristae maintenance, cause mitochondrial disease. Knock-in mice modeling the human CHCHD10S59L variant associated with ALS-FTD develop a mitochondrial cardiomyopathy driven by CHCHD10 aggregation and activation of the mitochondrial integrated stress response (mtISR). We show that cardiac dysfunction is associated with dual defects originating at the onset of disease: (1) bioenergetic failure linked to impaired mitochondrial copper homeostasis and cytochrome c oxidation, and (2) maladaptive mtISR signaling via the OMA1-DELE1-HRI axis. Using protease-inactive Oma1E324Q/E324Q knock-in mice, we show that blunting mtISR in Chchd10S55L/+ mice delays cardiomyopathy onset without rescuing CHCHD10 insolubility, cristae defects or OXPHOS impairment. Proteomic profiling of insoluble mitochondrial proteins in Chchd10S55L/+ mice reveals widespread disruptions of mitochondrial proteostasis, including IMS proteins involved in cytochrome c biogenesis. Defective respiration in mutant mitochondria is rescued by the addition of cytochrome c, pinpointing IMS proteostasis disruption as a key pathogenic mechanism. Thus, mutant CHCHD10 insolubility compromises metabolic resilience by impairing bioenergetics and stress adaptation, offering new perspectives for the development of therapeutic targets.