Link to Pubmed [PMID] – 20039420
Arthritis Rheum. 2010 Jan;62(1):268-79
OBJECTIVE: Skeletal muscle may be the site of a variety of poorly understood immune reactions, particularly after myofiber injury, which is typically observed in inflammatory myopathies. This study was undertaken to explore both the cell dynamics and functions of resident macrophages and dendritic cells (DCs) in damaged muscle, using a mouse model of notexin-induced myoinjury to study innate immune cell reactions.
METHODS: The myeloid cell reaction to notexin-induced myoinjury was analyzed by microscopy and flow cytometry. Bone marrow (BM) transplantation studies were used to discriminate resident from exudate monocyte/macrophages. Functional tests included cytokine screening and an alloantigenic mixed leukocyte reaction to assess the antigen-presenting cell (APC) function. Selective resident macrophage depletion was obtained by injection of diphtheria toxin (DT) into CD11b-DT receptor-transgenic mice transplanted with DT-insensitive BM.
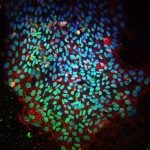
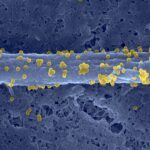
RESULTS: The connective tissue surrounding mouse muscle/fascicle tissue (the epimysium/perimysium) after deep muscle injury displayed a resident macrophage population of CD11b+F4/80+CD11c-Ly-6C-CX3CR1- cells, which concentrated first in the epimysium. These resident macrophages were being used by leukocytes as a centripetal migration pathway, and were found to selectively release 2 chemokines, cytokine-induced neutrophil chemoattractant and monocyte chemoattractant protein 1, and to crucially contribute to massive recruitment of neutrophils and monocytes from the blood. Early epimysial inflammation consisted of a predominance of Ly-6C(high)CX3CR1(low)CD11c- cells that were progressively substituted by Ly-6C(low)CX3CR1(high) cells displaying an intermediate, rather than high, level of CD11c expression. These CD11c(intermediate) cells were derived from circulating CCR2+ monocytes, functionally behaved as immature APCs in the absence of alloantigenic challenge, and migrated to draining lymph nodes while acquiring the phenotype of mature DCs (CD11c+Ia+CD80+ cells, corresponding to an inflammatory DC phenotype).
CONCLUSION: The results in this mouse model show that resident macrophages in the muscle epimysium/perimysium orchestrate the innate immune response to myoinjury, which is linked to adaptive immunity through the formation of inflammatory DCs.