Link to Pubmed [PMID] – 19823575
PLoS ONE 2009 Oct;4(10):e7375
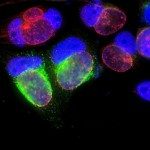
Pathogens use diverse molecular machines to penetrate host cells and manipulate intracellular vesicular trafficking. Viruses employ glycoproteins, functionally and structurally similar to the SNARE proteins, to induce eukaryotic membrane fusion. Intracellular pathogens, on the other hand, need to block fusion of their infectious phagosomes with various endocytic compartments to escape from the degradative pathway. The molecular details concerning the mechanisms underlying this process are lacking. Using both an in vitro liposome fusion assay and a cellular assay, we showed that SNARE-like bacterial proteins block membrane fusion in eukaryotic cells by directly inhibiting SNARE-mediated membrane fusion. More specifically, we showed that IncA and IcmG/DotF, two SNARE-like proteins respectively expressed by Chlamydia and Legionella, inhibit the endocytic SNARE machinery. Furthermore, we identified that the SNARE-like motif present in these bacterial proteins encodes the inhibitory function. This finding suggests that SNARE-like motifs are capable of specifically manipulating membrane fusion in a wide variety of biological environments. Ultimately, this motif may have been selected during evolution because it is an efficient structural motif for modifying eukaryotic membrane fusion and thus contribute to pathogen survival.