About
The symbiotic microbiota plays an essential role at many levels of host physiology, and germfree mice develop a large number of defects. In the intestine, the microbiota metabolises nutrients and toxins to generate an output that provides the hosts with vital compounds. The microbiota also regulates tissue homeostasis, provides protection against pathogens and affects the development and activation of the intestinal and systemic immune system. We have shown how compounds shed by the intestinal microbiota activates a cascade of signaling events that leads to the activation of intestinal LTi cells, the activation of stromal cells, the recruitment of B cells, and the formation of hundreds of tertiary lymphoid tissues involved in intestinal homeostasis (Bouskra, Nature 2008). We aim at understanding how the symbiotic microbiota affects the development and activation of pro-inflammatory immunity in particular (Sawa, Nat Immunol 2011), and of intestinal and systemic immunity in general, in order to establish a functional interactome between the microbiota, the immune system and the host.
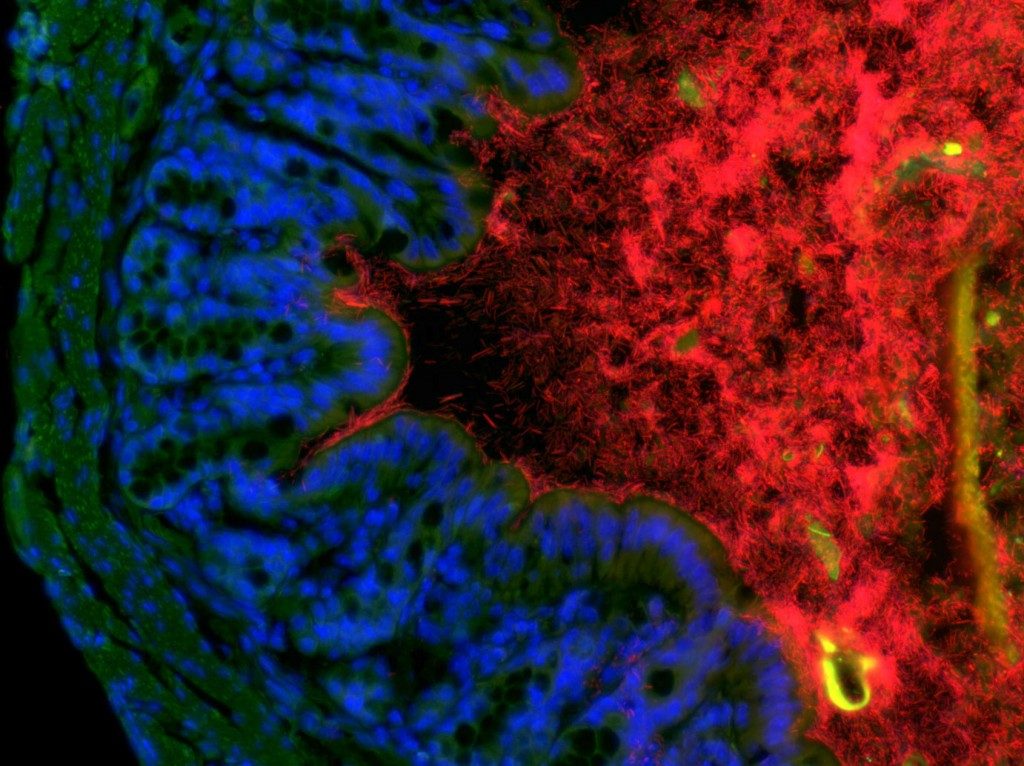
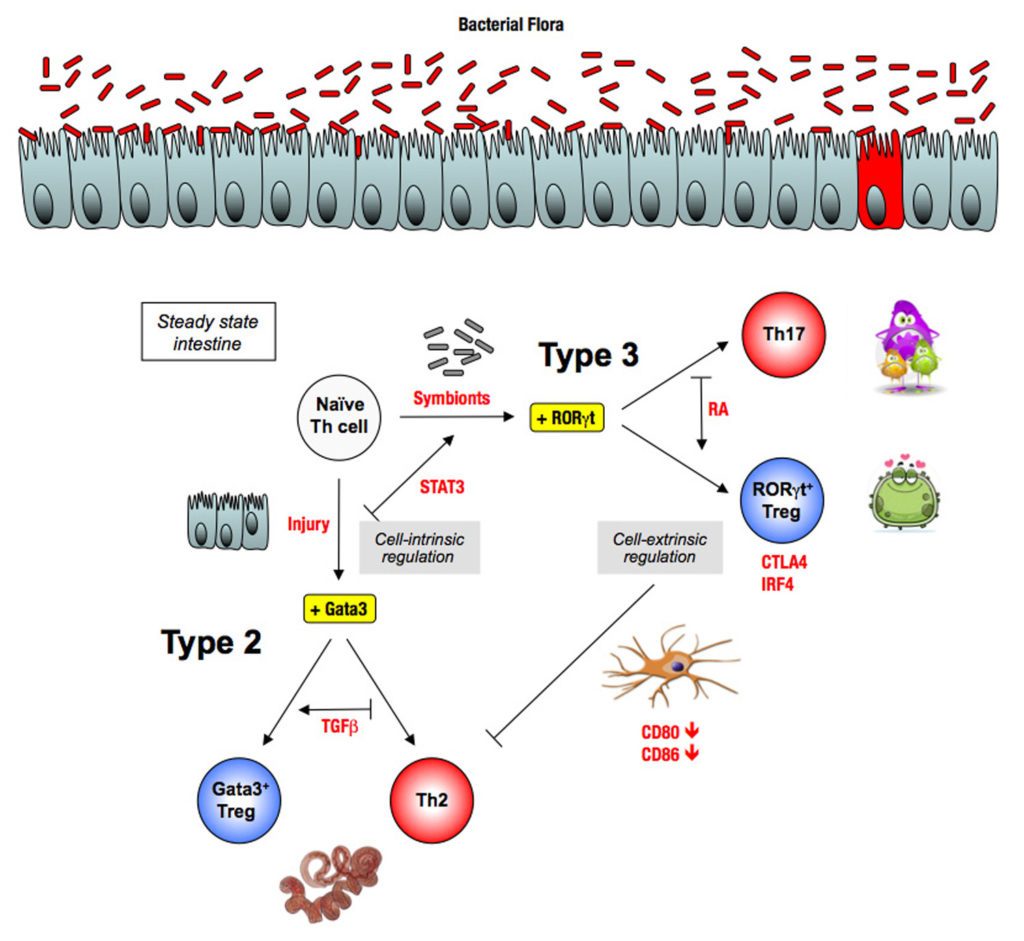
Recently, we have shown how the bacterial symbionts of the intestine regulate pro-allergic immune responses through the induction of type 3 Tregs (Ohnmacht, Science 2015).
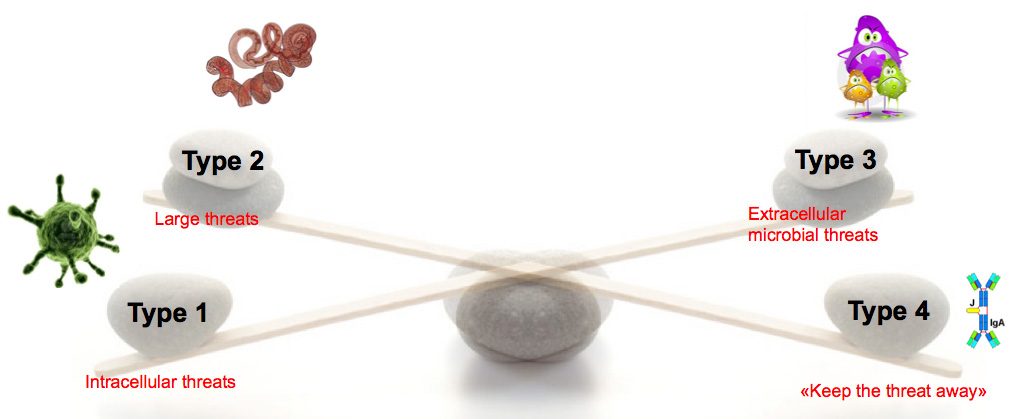
The underlying hypothesis of this line of experiments is the Equilibrium Model of Immunity, proposing that microbiota is necessary to maintain a healthy equilibrium between the different forces of the immune system. This equilibrium can be perturbed with potentially pathological consequences. For example, a virus infection induces a type 1 response that blocks the other types of responses, which can be detrimental upon a super-infection with another type of microbe. Conversely, the absence of one type of microbes, for example of bacteria upon antibiotic treatment, leads to decreased type 3 responses and as a consequence, exacerbated type 1 or type 3 responses.
Using different new mouse models to manipulate the immune system or the microbiota, we are investigating how the microbiota regulates this equilibrium, and how the immune equilibrium affects other systems, such as metabolism and brain.