About
Morphogenesis, that is the acquisition of a shape, is essential for organ function. The heart is an asymmetric organ, in which the left and right sides are fully separated to drive the double blood circulation in mammals. The molecular cascade breaking bilateral symmetry in the early embryo has been well characterised, with the formation of a left-right organiser, the node. However, how left-right asymmetric gene expression is later sensed by organ specific precursor cells to generate asymmetric morphogenesis is poorly understood. Our aim is to dissect how patterning of heart precursors drives asymmetric heart morphogenesis, and how anomalies in this process are associated with congenital heart defects.
In the early embryo, looping of the heart tube, is the first morphological sign of left-right asymmetry. It corresponds to the transformation of the initial straight heart tube into a helix. Heart looping is essential to align the cardiac chambers and great vessels and thus establish the plumbing of the blood flow. Previously, heart looping had been described as a binary process (rightward or leftward direction), corresponding to the readout of the symmetry-breaking event, but the fine 3D shape of the heart loop, as a readout of asymmetric morphogenesis had not been addressed. We thus propose a paradigm shift in the analysis of heart looping, from a binary process to spatio-temporal dynamics. This will be essential to uncover the pathophysiology of laterality defects such as heterotaxy, which are associated with a broad phenotypic variability.
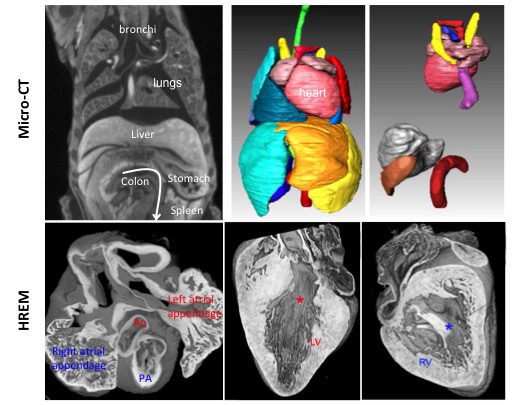
Using High Resolution Episcopic Microscopy (HREM), we have reconstructed mouse heart looping dynamics in 3 dimensions and developed tools to quantify the shape of the heart tube, not just its direction. The staging criteria that we have established provide a higher temporal resolution of early mouse heart development.
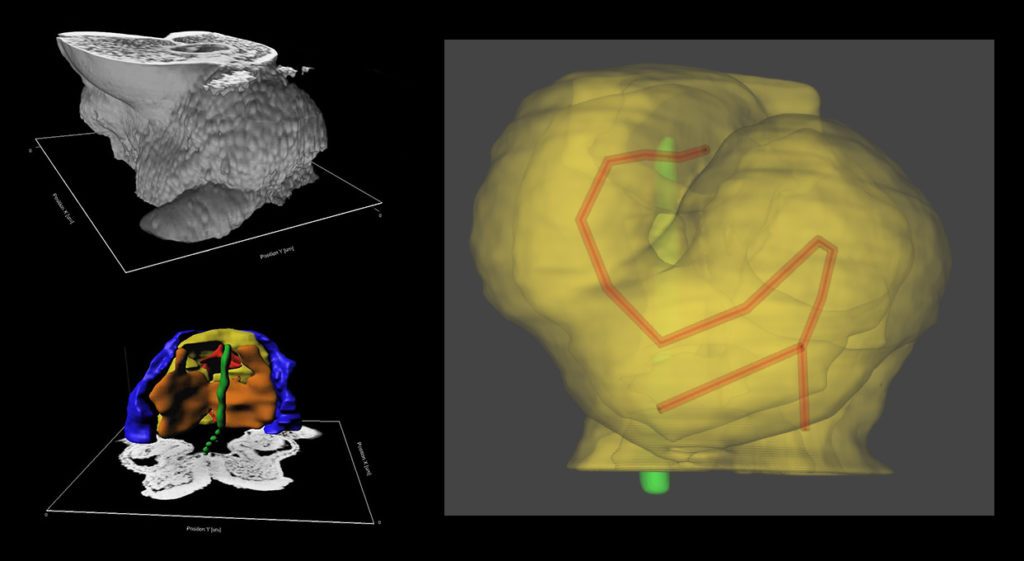
We have proposed a novel mechanism of heart looping and developed a computer model, to predict the shape of the looped heart tube from initial mechanical constraints and sequential left-right asymmetries.
Le Garrec et al., 2017 – Video of a computer simulation of heart looping
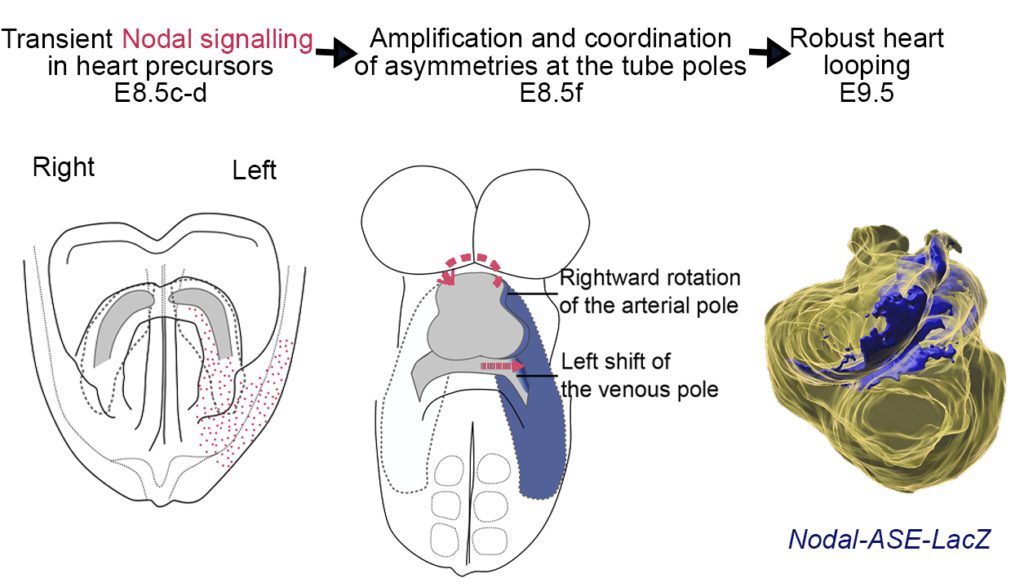
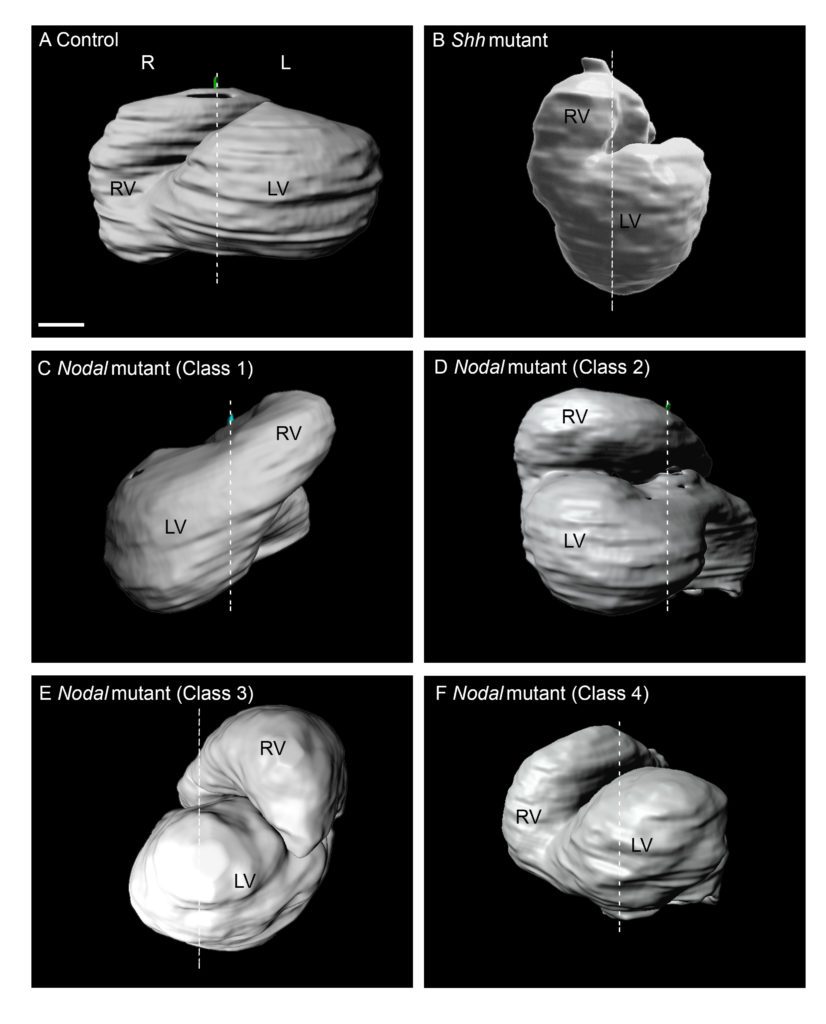
Recently, we have analysed how left-sided Nodal signalling, which is impaired in the heterotaxy syndrome, shapes the embryonic heart tube. We have shown that Nodal is not required to initiate heart looping, but rather to amplify and orient left-right asymmetries at the tube poles. We have dissected the spatio-temporal window of Nodal signalling in heart precursors, as well as its downstream effectors.
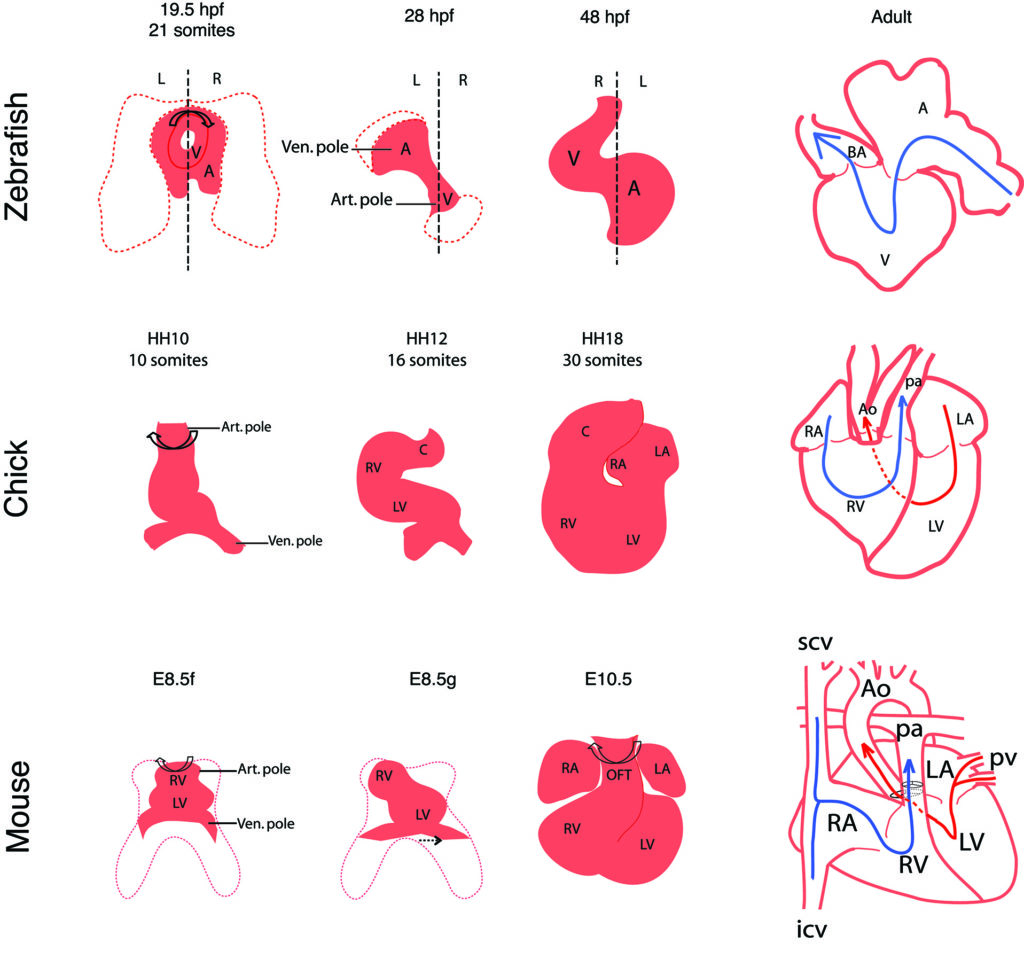
In a review, we have compared heart looping mechanisms and dynamics in the main vertebrate models : the fish, chick and mouse.
Our work on mouse heart morphogenesis is relevant to congenital heart defects in humans, such as misalignment of cardiac chambers. Congenital heart diseases represent a major concern for public health, affecting 1% of newborns and a leading cause of death in utero and during the first year of life. However, the genetic bases of these defects and the underlying pathophysiological processes remain poorly understood.
Phenotyping of laterality defects, such as heterotaxy, has been limited by fragmented observations. We have established a standardised procedure for a multiscale, multistage, 3D analysis of visceral organ asymmetry in their normal environment, together with that of the fine anatomy of left/right cardiac segments.