About
Cytokinesis requires a complex series of cytoskeletal changes, including remodeling of the actin, septin, and microtubule cytoskeleton. This is essential for the assembly and constriction of the ESCRT-III filaments that drives the final abscission.
Specifically, we are interested in understanding how actin filaments are cleared at the abscission site, how actin/septin/ESCRTs are coordinated and how changes in lipids can control abscission.
For instance, we discovered that the oncogenic Rab35 GTPase, through different effectors, plays a key role in cytokinetic abscission. This involves phosphoinositide lipid hydrolysis by the PI(4,5)P2 phosphatase OCRL —which is mutated in patient suffering from the Oculo-Cerebro-Renal syndrome of Lowe— and actin depolymerization by the oxidoreductase MICAL1 —an enzyme that specifically oxidizes actin filaments to induce their disassembly. Our work revealed the first connection between oxidoreduction and cell division by triggering local actin depolymerization and thereby allowing ESCRT filament constriction at the abscission site.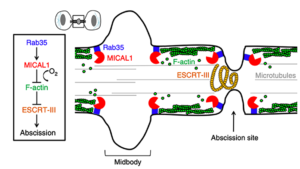
To learn more, see our past publications:
Kouranti et al. Current Biology 2006
Miserey-Lenkei et al. Nature Cell Biology 2010
Dambournet et al. Nature Cell Biology 2011
Chesneau et al. Current Biology 2012
Cauvin et al. Current Biolgy 2016
Klinkert et al. Nature Communications 2016
Fremont et al. Nature Communications 2017
Ribet et al. Journal of Cell Biology 2017
Mondin et al. Journal of Cell Biology 2019
Bai et al. PNAS 2020
Addi et al. Nature Communications 2020