About
Bovine Viral Diarrhea virus envelop protein E(rns)
Pestiviruses, which belong to the Flaviviridae family of RNA viruses, are important agents of veterinary diseases causing substantial economical losses in animal farming worldwide. Pestivirus particles display three envelope glycoproteins at their surface: E(rns), E1, and E2.
We report here the crystal structure of the catalytic domain of E(rns) of the Bovine Viral Diarrhea Virus (BVDV), the ribonucleolytic activity of which is believed to counteract the innate immunity of the host. The structure reveals a three-dimensional fold corresponding to T2 ribonucleases from plants and fungi. Co-crystallization experiments with mono- and oligonucleotides revealed the structural basis for substrate recognition at two binding sites previously identified for T2 RNases. A detailed analysis of poly-U cleavage products using 31P-NMR and size exclusion chromatography, together with molecular docking studies, provides a comprehensive mechanistic picture of Erns activity on its substrates and reveals the presence of at least one additional nucleotide binding site.
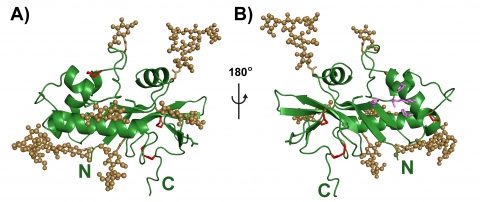
Structure of the E(rns) pestivirus glycoprotein from BVDV.
Erns is displayed as green ribbons, N- and C-terminus are labeled in green. Disulfide bridges are depicted as red sticks.
(A) View on the convex side of the molecule: Asparagine side chains of N-glycosylation sites and N-linked glycansare respectively in light brown sticks and spheres.
(B) View on the concave face of the molecule: Putative active site of the enzyme is in purple.The N-linked glycans cluster on the convex face of the molecule.
Crystal structure of the Pestivirus Envelope Glycoprotein E(rns) and Mechanistic Analysis of Its Ribonuclease Activity. Krey T, Bontems F, Vonrhein C, Vaney MC, Bricogne G, Rümenapf T, Rey FA. Structure. 2012 May 9;20(5):862-73.
This work was supported by the French ‘Agence Nationale de Recherche’ (ANR, 05 MIIM 012 01), by Merck-Serono and recurrent funding from the Institut Pasteur and the CNRS. K.T. benefited from an Institut Pasteur ‘‘Bourse Roux’’ and then from an EU Marie Curie fellowship (MEIF-CT-2007-04725).



