About
The mechanisms governing ageing, which is a multifactorial process, have not been resolved and constitute a fundamental open question in cell and organismal biology. Ageing is dramatically accelerated in some rare genetic disorders. Dissecting the defect(s) of these diseases is critical to develop treatments and elucidate dysfunctions that might lead to normal ageing. The progeroid Cockayne syndrome (CS) is characterized by severe neurodevelopmental defects, dramatically precocious ageing, and in most cases clinical photosensitivity.
By assessing patient-derived primary cells we showed that CS alterations are rather linked to impairment of the mitochondrial DNA replication complex, in particular depletion of the catalytic mitochondrial DNA polymerase. This alteration affects mitochondrial ATP production, and is due to accumulation of the HTRA3 serine protease. HTRA3 is in turn overexpressed by increased nitroso-oxidative stress. We rescued mitochondrial alterations in CS patient cells with drugs that act on serine proteases or scavenge reactive oxygen and nitrogen species, opening novel possibilities for treatments, which are presently lacking for CS patients. We have also demonstrated links between CS molecular defects and replicative senescence, a processes linked to physiological ageing.
We investigate genetic and epigenetic mechanism(s) by which CSA/B impairment promotes HTRA3 overexpression, the extent of mitochondrial and cellular protein depletion upon HTRA3 overexpression, and the occurrence of these events during normal ageing. We also investigate large-scale epigenomic and gene expression changes that are linked or causative of the accelerated ageing phenotype with possible implications also for physiological ageing.
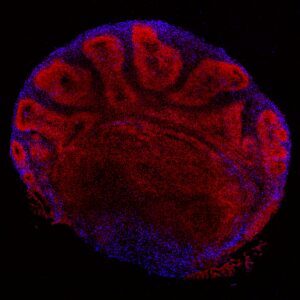
CS displays large clinical heterogeneity, the reason of which is not known, and cannot be explained by simple genotype/phenotype correlations. Our working hypothesis is that CS heterogeneity is largely due to the diverse management of oxidative/nitrosative stress, and to mitochondrial metabolites that are intermediate of epigenetic regulators of nuclear gene expression. To disentangle the effect of the mutation per se, using the CRISPR/Cas9 technology we have constructed isogenic cellular models with mutations responsible from severe or mild forms of CS. As animal models poorly recapitulate the disease, and patient material is rare and mostly unavailable, we generated induced pluripotent stem cells (iPSCs) from cells of patients with different clinical severity. From iPSCs we also derive differentiated cells and cerebral organoids (COs) to assess molecular, cellular, and intercellular alterations that are are linked to the accelerated ageing-related degeneration in this disease. Our project is at the intersection of progeroid diseases and physiological ageing.