About
CyaA-based therapeutic cancer vaccines
Based on our discovery that a bacterial protein, the adenylate cyclase (CyaA), binds to dendritic cells (DCs), we have developed a highly efficient vector capable of targeting a wide range of antigens to antigen presenting cells (APC), leading to strong immune responses. A large panel of recombinant CyaAs carrying different heterologous viral and/or tumoral T-cell epitopes were shown to stimulate strong and specific Th1 and CTL responses in animals, and to induce protective immunity against viral and tumoral challenges, as well as therapeutic efficacy against transplanted tumors. We recently demonstrated that CyaA possesses the intrinsic ability not only to target DCs but also to activate them through the TLR4/TIR domain containing adapter-inducing IFN-ß (TRIF) pathway, leading to the induction of strong immune responses (Dadaglio et al, J. Immunol, 2014). We used this new vector to develop two therapeutic vaccine candidates against cervical cancer (ProCervix) and melanoma (Theravac) which have entered in clinical trials. ProCervix, based on the CyaA vector, is a bivalent HPV therapeutic vaccine candidate currently evaluated in a large Phase I clinical trial for women who are infected with HPV 16 and/or HPV 18, before appearance of high grade cervical lesions. Clinical phase I trial results in 47 patients indicate good safety and local tolerance at the highest dose evaluated. Antigen-specific T cell responses have been detected in a majority of vaccinated women. Moreover, viral clearance is markedly more frequent in patients treated with ProCervix than in the placebo group. In addition, only the group of patients vaccinated with ProCervix controlled HPV recurrence (http://www.genticel.com/). 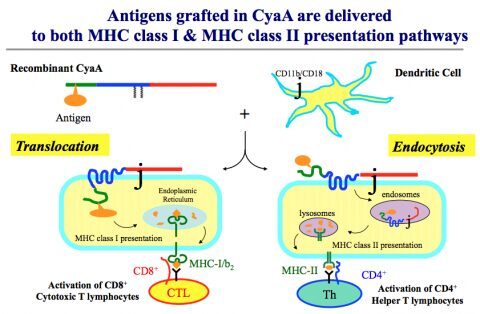
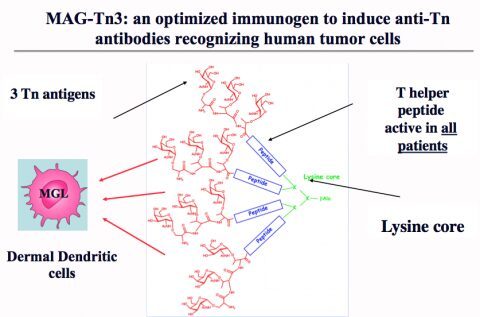
The MAG-Tn3 vaccine
A hallmark of cancer cells is a change in glycosylation processes leading to the abnormal expression of carbohydrate chains, such as antigens of the T family (Tn, sialyl-Tn and T) on most carcinomas (breast, colon, prostate and ovary). We have developed an efficient approach based on a fully synthetic glycopeptide structure, for the induction of antibodies against the Tn glycosidic antigen expressed by tumor cells.