About
Cell-cell fusion proteins are essential in development. We show that the C. elegans cell-cell fusion protein EFF-1 is structurally homologous to viral class II fusion proteins. The 2.6 Ang. crystal structure of the EFF-1 trimer displays the same 3D fold and quaternary conformation of postfusion class II viral fusion proteins, although it lacks a nonpolar ‘‘fusion loop,’’ indicating that it does not insert into the target membrane. EFF-1 was previously shown to be required in both cells for fusion, and we show that blocking EFF-1 trimerization blocks the fusion reaction.
The structural data reported for a genuine cell-cell fusion protein unambiguously demonstrate an evolutionary link with viral fusion proteins. They constitute a further illustration of the extensive genetic exchanges between viruses and cells throughout evolution and of the striking adaptation of a protein to maintain the same function while adopting an altered mode of action. These data now open the way to a full mechanistic characterization of membrane fusion induced by FF proteins. Our results thus raise a number of new questions, such as the o ganization of the TM segments in the postfusion trimer, the structure of the prefusion form and its organization on membranes, and how the proposed trans-trimerization fusogenic process is triggered.
Finally, these findings provide additional elements that can help in identifying an as yet elusive sequence signature for the class II protein fold and will therefore stimulate the use of structural homology searches to discover unidentified fusion protein homologs in other eukaryotic organisms, for instance, in mammals.
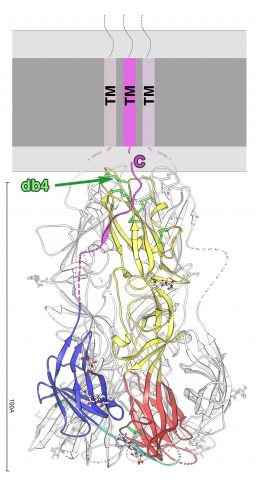
Structure of the EFF-1 trimer protein.
Ribbon diagram of a subunit of the EFF-1 trimer, colored according to the class II convention:red, yellow, and blue for domains I, II, and III, respectively.The green arrow points to the conserved class II protein disulfide bond.
Structural basis of eukaryotic cell-cell fusion. Perez-Vargas J, Krey T, Valansi C, Avinoam O, Haouz A, Jamin M, Raveh-Barak H, Podbilewicz B, Rey FA. Cell. 2014 Apr 10;157(2):407-19.
This work was funded by the French ‘‘Agence Nationale pour la Recherche’’ grant ANR-2010-BLAN-1211 01 and by Institut Pasteur, CNRS, and Merck-Serono.

