About
AdipoTryp
Trypanosoma brucei is a unicellular and extracellular parasite transmitted by the tsetse fly (Glossina genus) and causing Human African Trypanosomiasis (HAT) in Sub-Saharan Africa. If untreated, this neglected tropical disease has a case fatality rate close to 100%. There is no vaccine and the currently available drugs present significant side-effects, variable efficacies and/or do not cross the blood brain barrier. Other related parasite species are responsible for animal African trypanosomiases that remain a major constraint to productive livestock rearing in countries throughout Sub-Saharan Africa, causing major economic losses.
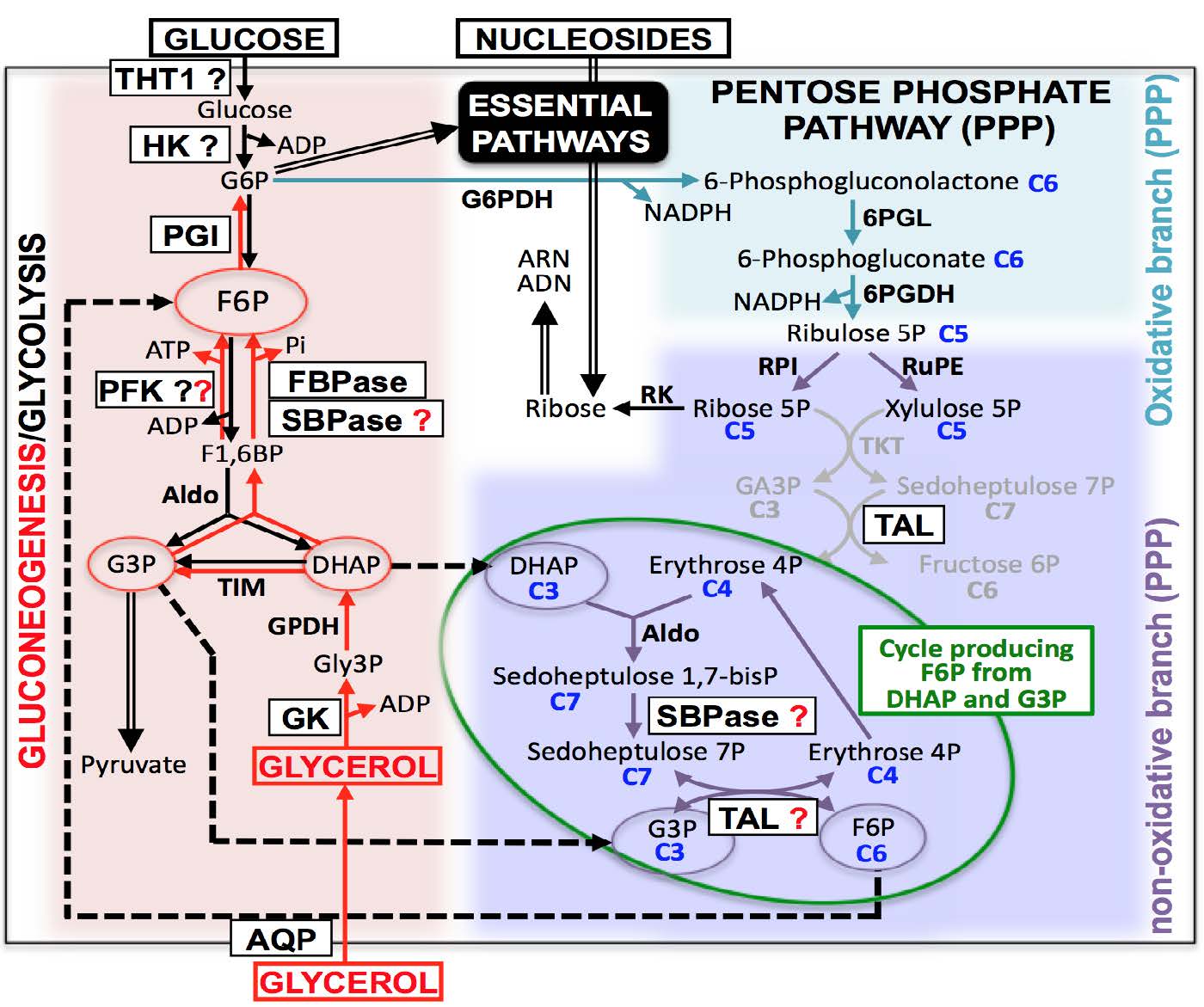
It has been considered for decades that trypanosomes propagate exclusively in the body fluids of their mammalian hosts and mostly in the blood. In a break with this dogma, three recent publications showed that most parasites actually reside in the extravascular compartment of mouse models, especially in the adipose tissues and the skin, from which transmission can occur. Within the skin, some parasites seem to tightly interact with adipocytes, the major constituent of fat, suggesting that the adipocytes-trypanosomes interactions might confer a selective advantage to the parasites. In resonance with this major discovery, we recently broke another strong dogma considering that T. brucei relied exclusively on the glucose provided by the mammalian host blood to feed its central carbon metabolism. Indeed, we established long-term conditions for growth of the parasite in glucose-free medium containing glycerol. Since adipocytes excrete large amounts of glycerol from lipolysis and glycolysis, we hypothesised that extravascular trypanosomes could take advantage of this glycerol production to (i) feed their central carbon metabolism and (ii) colonize the glycerol-rich tissues by chemotactism. Our preliminary data presented in details in the application support these two hypotheses.
The objectives of this project are (i) to evaluate the importance of the glycerol metabolism for parasites in vivo, including a potential role of the glycerol as a metabolic sensor to attract trypanosomes in specific tissues, (ii) to develop an in vitro assay to study the metabolic interactions between adipocytes and trypanosomes, (iii) to determine the probable alternative(s) to FBPase, the key gluconeogenic enzyme, as well as the role of the parasite gluconeogenesis in vivo in the mammalian host, (iv) to confirm the existence and determine the role of b-oxidation of fatty acids previously proposed to be activated in tissue-dwelling parasites, and (v) to identify the most relevant metabolic step(s) targeted by suramin. All these questions will be tackled by leading experts in complementary fields forming a long-standing network.
This international ANR project involving 5 partners supervised by F. Bringaud at the Univerity of Bordeaux will have major impacts in understanding the biology of tissue trypanosomes. It will also pave the way for the development of therapeutic approaches targeting extravascular parasites.