The pathogen Legionella pneumophila modulates the biochemistry of the cell it infects to its own benefit. The bacterium stops mitochondrial respiration, while maintaining the membrane potential of the mitochondria to delay the destruction of the cell.
L. pneumophila is a bacterial pathogen that causes a severe pneumonia called Legionellosis, which is often fatal when not treated promptly. In France, between 1200 and 2000 cases are identified each year, with mortality rates ranging from 5 to 15%. A recent study conducted by scientists at the Institut Pasteur and published in eLife shows that this bacterium is able to modulate the action of a cellular enzyme to its own advantage.
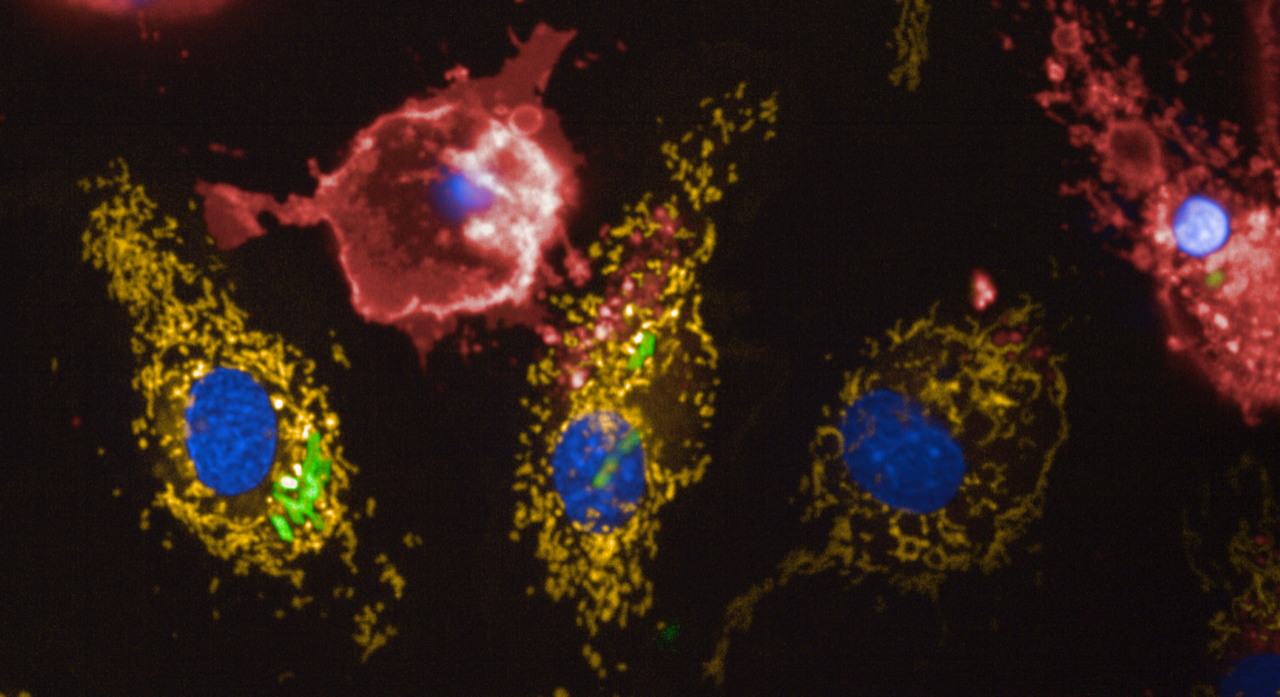
Legionella targets the mitochondria of its host cell. L. pneumophila is an intracellular pathogen that has evolved unique strategies to proliferate intracellularly by subverting the defenses of the host cell. Importantly, L. pneumophila targets different host organelles, among those mitochondria. Mitochondria are well-defined cytoplasmic organelles that have many important functions. In particular, they supply the energy to the cell. Previously, researchers at the Institut Pasteur have shown that L. pneumophila modulates mitochondrial dynamics by inducing their fission. A consequence of this fragmentation of the mitochondrial network is that it impairs mitochondrial respiration, a sequence of reactions that generate and use the mitochondrial membrane potential to synthesize ATP, a very important molecule driving the energy in the cell.
However, a question remained unanswered. Despite the bacterial-induced mitochondrial fission, host cell mitochondria maintain their normal membrane potential during infection. A mitochondrial membrane potential is generated during respiration under normal conditions, but L. pneumophilastops mitochondrial respiration, how is it possible that mitochondria have normal membrane potential in the absence of respiration?
Maintaining the characteristics of mitochondria to preserve the bacterium’s replication niche
In this new study, researchers from the Biology of Intracellular Bacteria unit at the Institut Pasteur highlight that L. pneumophila infection modifies the activity of a mitochondrial enzyme, the FOF1-ATPase, to function in “reverse mode” instead of the “forward mode”. This thereby maintains the normal membrane potential in the absence of mitochondrial respiration. L. pneumophila is known for its ability to translocate cocktails of proteins into its eukaryotic host that modulate numerous host defense pathways. This study shows that one of these bacterial protein transferred into the host cell, LpSpl, participates in the process of changing the activity of the mitochondrial FOF1-ATPase.
The bacterial induced change of the mode of function of this mitochondrial ATP synthase leads to delayed cell death. Indeed, a decrease in the membrane potential of the mitochondria is a trigger of cell death. Therefore, as L. pneumophila stops mitochondrial respiration in the cell, this mechanism maintains the potential and delays the onset of cell death of the host. Ultimately, this supports bacterial replication by preserving its replication niche. This work sheds new light on how a pathogen modulates host organelles and the function of their enzymes during infection, ultimately allowing better replication in human cells and thus to cause disease.
Source:
Reverting the mode of action of the mitochondrial FOF1-ATPase by Legionella pneumophila preserves its replication niche, eLife, December 9, 2021
 Pedro Escoll1,2, Lucien Platon1,2,3, Mariatou Dramé1,2,4, Tobias Sahr1,2,
Pedro Escoll1,2, Lucien Platon1,2,3, Mariatou Dramé1,2,4, Tobias Sahr1,2,
Silke Schmidt1,2,5, Christophe Rusniok1,2, Carmen Buchrieser1,2
1 – Institut Pasteur, Biologie des Bactéries Intracellulaires, Paris, France
2- Institut Pasteur, Biologie des Bactéries Intracellulaires and CNRS UMR 3525, Paris, France
3 – Faculté des Sciences, Université de Montpellier, Montpellier, France
4 – Faculté des Sciences, Université de Paris, Paris, France
5 – Sorbonne Université, Collège doctoral, Paris, France