The topological reorganization imposed on the chromatin during mitosis leads to a global shutdown of the gene expression. How then is the transcriptional program reestablished after division ? Previous work lacks the coupling between spatial and temporal resolution to assess in real-time the interplay between the transcriptional machinery and the physical properties of the chromatin. The goal of this project is to investigate gene regulation with high spatial and temporal resolution before, during and just after mitosis. Using quantitative imaging we will monitor enhancer-promoter contacts and continuously record transcriptional activity, particularly as cells undergo mitosis. Computational and statistical analyses as well as polymer models will be developed to quantify dynamic interactions and associate them to TF activity, mitotic progression and transcriptional outputs.
© Melody Merle
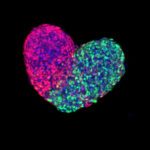
Fluorescently labeled five day old gastruloid, a mouse embryonic stem-cell derived pseudo-embryo.