About
All known proteins required for cytokinetic abscission are enriched in the midbody, the central part of the intercellular bridge that connects the daughter cells after furrow ingression. We and others discovered that, in most instances, abscission occurs on both sides of the midbody releasing a midbody remnant (MBR) that is tightly attached to the cell surface before being engulfed usually by one of the daughter cells and sometimes by distant cells.
We achieved the purification of highly pure MBRs from cancer cells and obtained their quantitative proteome by mass spectrometry that we named the “Flemmingsome”. Proteins that are enriched in the MBR are excellent candidates for playing a role in cytokinesis. As a proof of concept, detailed functional analysis demonstrated the role of Syndecan 4 (SDC4) in coupling the ESCRT-III machinery to the plasma membrane through interactions with syntenin and ALIX. This revealed the first transmembrane proteins directly involved in cytokinetic abscission.
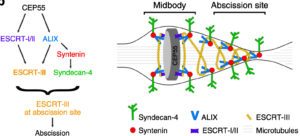 Working model: ALIX-syntenin-syndecan-4 couples the ESCRT-III machinery to the plasma membrane at the abscission site for efficient scission. ALIX-syntenin (blue-red), by directly coupling ESCRT-III (yellow) filaments on the one hand and the transmembrane protein syndecan-4 (green) on the other hand is proposed to help maintain ESCRT-III polymers at the abscission site until the final cut.
Working model: ALIX-syntenin-syndecan-4 couples the ESCRT-III machinery to the plasma membrane at the abscission site for efficient scission. ALIX-syntenin (blue-red), by directly coupling ESCRT-III (yellow) filaments on the one hand and the transmembrane protein syndecan-4 (green) on the other hand is proposed to help maintain ESCRT-III polymers at the abscission site until the final cut.
Based on the Flemmingsome, we also revealed the first role of caveolae in cell division. Caveolae are small, mechanosensitive invaginations of the plasma membrane known since 1950’s. We found pools of caveolae at the midbody, abscission site and interfaces between the cytokinetic bridge and the cell bodies. Caveolae help to release mechanical tension and thereby promote ESCRT machinery constriction and promote abscission.
We are working on many more hits that promote abscission both independently and in concert with ESCRT machinery.
We created for the community a database of the midbody remnant proteome, with regularly updated information on protein localization and function during cytokinesis. Please, visit https://flemmingsome.pasteur.cloud/
Please, visit https://flemmingsome.pasteur.cloud/
To learn more, see our past publications:
Addi et al. Nature Communications 2020
Andrade et al. Science Advances 2022