About
This project aims to identify key aspects of the viral replication cycle in terminally differentiated macrophages which are one of the major targets of HIV-1 using fluorescence microscopy approaches, quantitative image analysis and state-of-the art fluorescent labelling techniques. We focused our efforts to address the following aspects of the HIV-1 early infection cycle: in order to replicate, the Human Immunodeficiency Virus (HIV-1) reverse transcribes its RNA genome into DNA, which subsequently integrates into host cell chromosomes. These two key events of the viral life cycle are viewed as separate not only in time but also in cellular space, since reverse transcription (RT) is thought to be completed in the cytoplasm before nuclear import and integration. However, the spatiotemporal organization of the early replication cycle in macrophages, natural non-dividing target cells that constitute important reservoirs of HIV-1 and an obstacle to curing AIDS, remains unclear. In our study we demonstrated that infected macrophages display large nuclear foci of viral DNA and viral RNA, in which multiple genomes cluster together. These clusters form in the absence of chromosomal integration, displace the paraspeckle protein CPSF6 and localize to potential speckles because enriched in SC35. Strikingly, we show that viral RNA foci consist mostly of genomic, incoming RNA in which RT can resume after temporary inhibition of RT and nuclear import.
We propose to unravel the role and the underlying mechanism of CPSF6 cluster formation in HIV-1 infection in human macrophages.
We hypothesized that viral clusters could act as micro-reactors that locally concentrate viral and host cellular factors required for viral replication and/or to protect the virus from cellular defense mechanisms. Another possibility is that this is a self-defense strategy developed by the cell against the viral attack.
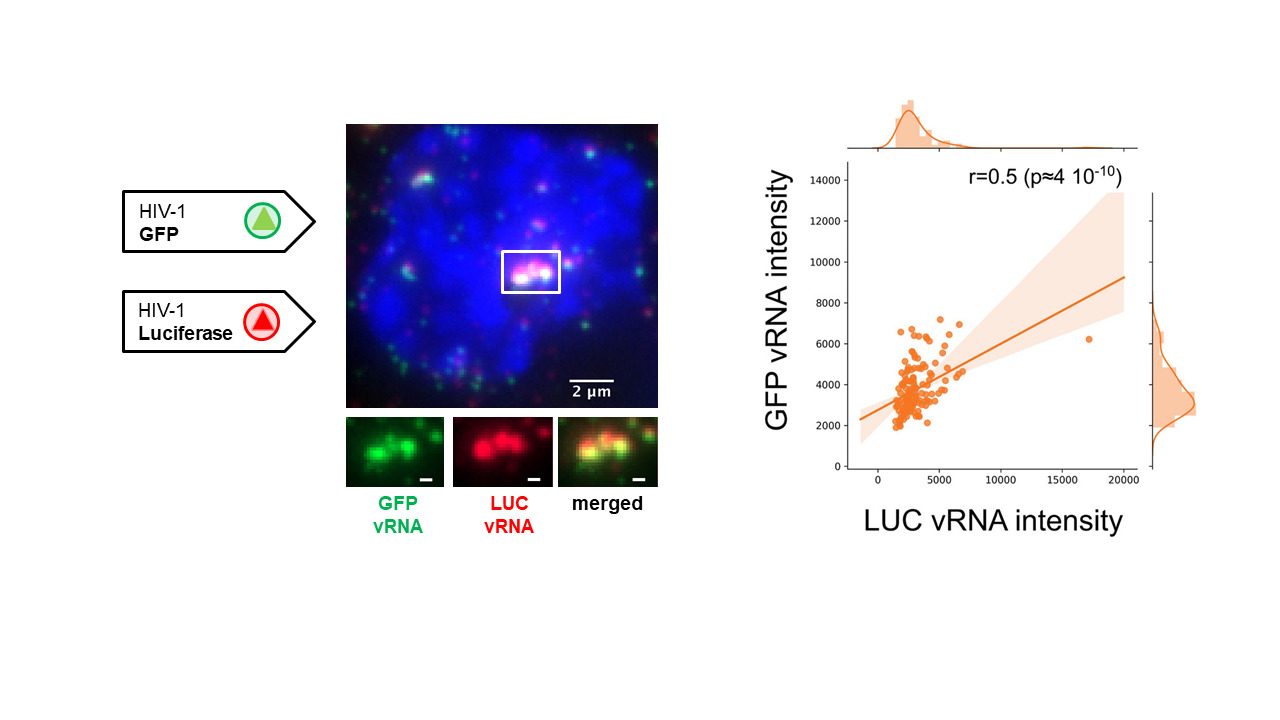
Reference: E. Rensen, F.Mueller, V. Scoca, J. Parmar, P. Souque, C. Zimmer, F. Di Nunzio “HIV-1 genomes cluster in nuclear niches of human macrophages” EMBO J. 2021.
Future studies from our group will focus to understand the viral and host factors that participate to the viral post-nuclear entry steps.