Link to Pubmed [PMID] – 10873442
Link to HAL – Click here
Link to DOI – 0.1006/jmbi.2000.3792
J Mol Biol. 2000 Jun 23;299(5):1157-64.
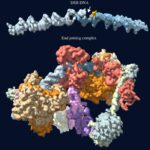
The crystal structure of aspartyl-tRNA synthetase from Escherichia coli has been determined to a resolution of 2.7 A. The structure is compared to the same enzyme co-crystallized with tRNA(Asp) and containing aspartyl adenylate or ATP. The asymmetric unit contains three monomers of the enzyme. While most parts of the protein show no significant differences in the three monomers, a few regions cannot be superimposed. Those regions are characterized by a high B-factor, and consist mostly of loops that make contacts with the tRNA in the complexes. The flexibility of the protein is seen at a global level, by the observation of a 10 to 15 degrees rotation of the N-terminal and insertion domains upon tRNA binding, and at the level of the individual amino acid residues, by main-chain and side-chain rearrangements. In contrast to these induced-fit conformational changes, a few residues essential for the tRNA anticodon or aspartyl-adenylate recognition exist in a predefined conformation, ensured by specific interactions within the protein.