Lien vers Pubmed [PMID] – 21558434
MBio 2011;2(3):e00043-11
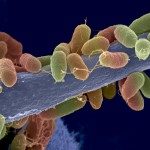
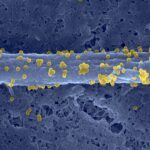
UNLABELLED: Bacterial biofilms often form multispecies communities in which complex but ill-understood competition and cooperation interactions occur. In light of the profound physiological modifications associated with this lifestyle, we hypothesized that the biofilm environment might represent an untapped source of natural bioactive molecules interfering with bacterial adhesion or biofilm formation. We produced cell-free solutions extracted from in vitro mature biofilms formed by 122 natural Escherichia coli isolates, and we screened these biofilm extracts for antiadhesion molecules active on a panel of Gram-positive and Gram-negative bacteria. Using this approach, we showed that 20% of the tested biofilm extracts contained molecules that antagonize bacterial growth or adhesion. We characterized a compound, produced by a commensal animal E. coli strain, for which activity is detected only in biofilm extract. Biochemical and genetic analyses showed that this compound corresponds to a new type of released high-molecular-weight polysaccharide whose biofilm-associated production is regulated by the RfaH protein. We demonstrated that the antiadhesion activity of this polysaccharide was restricted to Gram-positive bacteria and that its production reduced susceptibility to invasion and provided rapid exclusion of Staphylococcus aureus from mixed E. coli and S. aureus biofilms. Our results therefore demonstrate that biofilms contain molecules that contribute to the dynamics of mixed bacterial communities and that are not or only poorly detected in unconcentrated planktonic supernatants. Systematic identification of these compounds could lead to strategies that limit pathogen surface colonization and reduce the burden associated with the development of bacterial biofilms on medical devices.
IMPORTANCE: We sought to demonstrate that bacterial biofilms are reservoirs for unknown molecules that antagonize bacterial adhesion. The use of natural strains representative of Escherichia coli species biodiversity showed that nonbiocidal antiadhesion polysaccharides are frequently found in mature biofilm extracts (bacterium-free suspensions which contain soluble molecules produced within the biofilm). Release of an antiadhesion polysaccharide confers a competitive advantage upon the producing strain against clinically relevant pathogens such as Staphylococcus aureus. Hence, exploring the biofilm environment provides a better understanding of bacterial interactions within complex communities and could lead to improved control of pathogen colonization.