Lien vers Pubmed [PMID] – 35649238
Lien DOI – 10.1021/acschembio.1c00959
ACS Chem Biol 2022
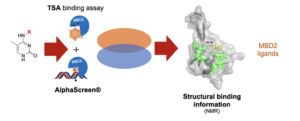
Epigenetics has received much attention in the past decade. Many insights on epigenetic (dys)regulation in diseases have been obtained, and clinical therapies targeting them are in place. However, the readers of the epigenetic marks are lacking enlightenment behind this revolution, and it is poorly understood how DNA methylation is being read and translated to chromatin function and cellular responses. Chemical probes targeting the methyl-CpG readers, such as the methyl-CpG binding domain proteins (MBDs), could be used to study this mechanism. We have designed analogues of 5-methylcytosine to probe the MBD domain of human MBD2. By setting up a protein thermal shift assay and an AlphaScreen-based test, we were able to identify three fragments that bind MBD2 alone and disrupt the MBD2-methylated DNA interactions. Two-dimensional NMR experiments and virtual docking gave valuable insights into the interaction of the ligands with the protein showing that the compounds interact with residues that are important for DNA recognition. These constitute the starting point for the design of potent chemical probes for MBD proteins.