Lien vers Pubmed [PMID] – 33926955
Lien vers HAL – pasteur-03228896
Lien DOI – 10.1126/science.abe6494
Science, 2021, 372 (6541), pp.516-520. ⟨10.1126/science.abe6494⟩
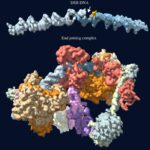
Cells have two purine pathways that synthesize adenine and guanine ribonucleotides from phosphoribose via inosylate. A chemical hybrid between adenine and guanine, 2-aminoadenine (Z), replaces adenine in the DNA of the cyanobacterial virus S-2L. We show that S-2L and Vibrio phage PhiVC8 encode a third purine pathway catalyzed by PurZ, a distant paralog of succinoadenylate synthase (PurA), the enzyme condensing aspartate and inosylate in the adenine pathway. PurZ condenses aspartate with deoxyguanylate into dSMP (N6-succino-2-amino-2′-deoxyadenylate), which undergoes defumarylation and phosphorylation to give dZTP (2-amino-2′-deoxyadenosine-5′-triphosphate), a substrate for the phage DNA polymerase. Crystallography and phylogenetics analyses indicate a close relationship between phage PurZ and archaeal PurA enzymes. Our work elucidates the biocatalytic innovation that remodeled a DNA building block beyond canonical molecular biology.