Présentation
Within cells, proteins are the real effectors of all activities. The expression of certain proteins changes upon cell stimulation or stress (with virus, inhibitors or bacteria), or when cells differentiate or turn into a disease state. Measuring the protein expression levels as well as characterizing their post-translational modifications (such as glycosylation, phosphorylation …) provides information on their cellular state. Overall, the goal of quantitative proteomics is to systematically study static state or perturbation-induced changes in protein profile. To achieve this goal, the proteomics platform uses two different strategies: i) label or label-free shotgun proteomics and ii) targeted proteomics using PRM (Parallel Reaction Monitoring).
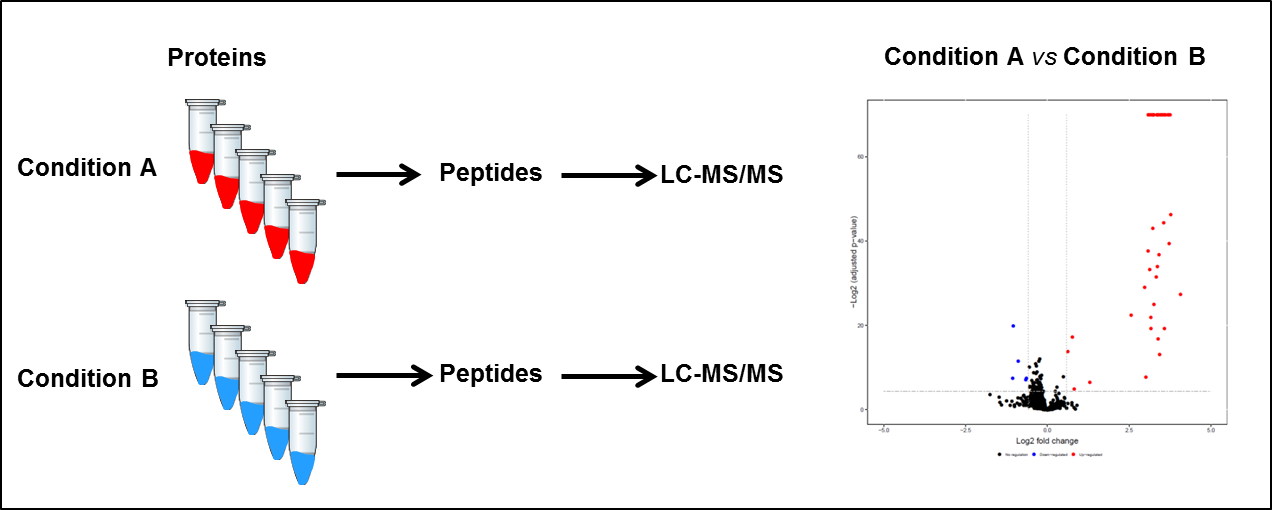
Project description 1: Label-free quantification
In this workflow, the protein content of a biological sample in different conditions (A and B for example) undergoes a tryptic digestion and the resulting peptides are analyzed by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Multiple samples can be analyzed sequentially and quantification is achieved by comparing the individual runs with each other using sophisticated tools and statistical analysis.
Project description 2: Targeting proteomics using Parallel Reaction Monitoring (PRM)
This workflow is used for the absolute quantification of a few selected proteins in a complex mixture. In contrast to shotgun proteomics described above, targeted proteomics is deterministic and is able to monitor the fragments of an analyte with very high sensitivity and specificity across the whole chromatographic elution of the LC from a complex mixture. It is thus often used if reproducible and accurate quantification is required across a large number of samples (such as a biomarker study). The proteomics platform recently acquired a new mass spectrometer, Q Exactive™ hybrid quadrupole Orbitrap™ MS, that allows a quantification of peptides using the latest advances in targeted LC-MS/MS analysis based on parallel reaction monitoring (PRM). This method allows the quantification of several proteins (up to 100) with high specificity, selectivity and sensitivity.