We tend to assume that programmed cell death and apoptosis should be negligible during the growth phase of a tissue. After all, what would be the point of “wasting” cells when there is a need for rapid cell proliferation to increase the size of the tissue/organ? As a result, the contribution of cell death to the final shape of growing organs and appendages has rarely been systematically explored .
This includes as well the wing imaginal disc, a small epithelial sac rapidly growing in the larvae of the fruit fly Drosophila and eventually giving rise to the adult wing. Despite decades of work to characterise the regulation of wing growth and shape, the role of cell death in this system has been overall neglected, assuming it was negligible and has no specific distribution in the tissue.
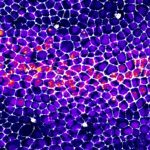
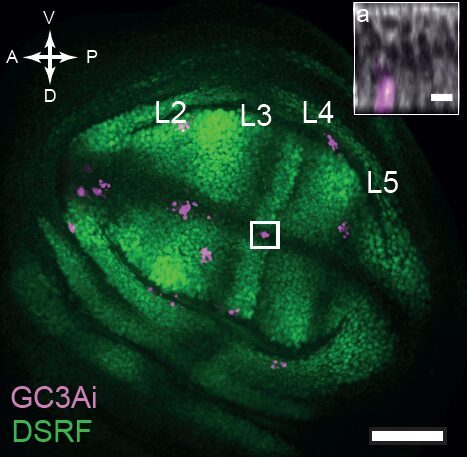
But is this really the case? Alexis Matamoro-Vidal and colleagues recently decided to re-examine this question using new methods to obtain a precise and quantitative mapping of cell death. It is usually very difficult to detect with confidence spatial patterns when looking at sporadic events (see for instance, the image below of a wing disc with cell death marked in magenta, where admittedly no one can really see a clear pattern).
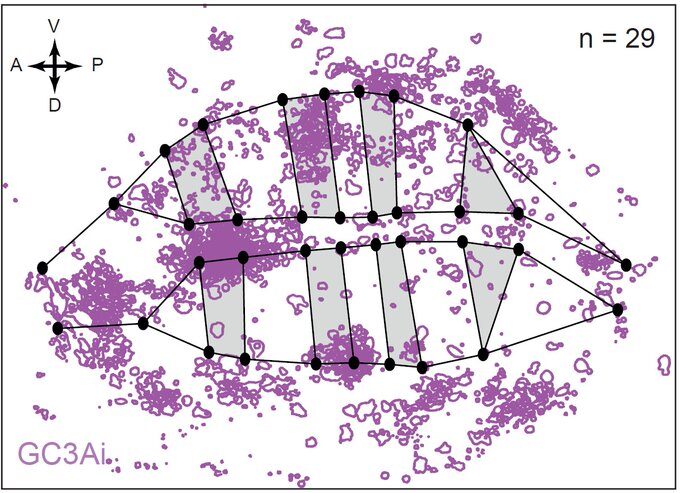
But what if one now observes a large number of tissues and systematically superimpose them using precise spatial landmarks? Much to our surprise, we obtained maps similar to this one showing cell death figures superimposed on 29 tissues.
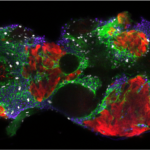
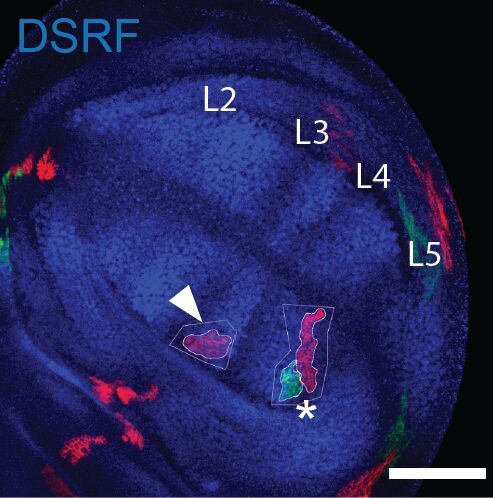
This was clearly outlining the existence of regions with a much higher incidence of cell death that had never been described before. Having confirmed this pattern with several markers, we wanted to check whether these “hotspots” of apoptosis could locally influence growth and survival of groups of cells. Using classical Drosophila genetic techniques to trace a mother cell and all its progeny (generating a so-called “clone”), we exploit tools allowing to mark the two daughter cells and their lineage with different colours after a few days. Normally, two neighbouring patches of different colours (here green and red) should always be observed… except when by chance one of the two initial daugther cell has died (see the white arrowhead below).
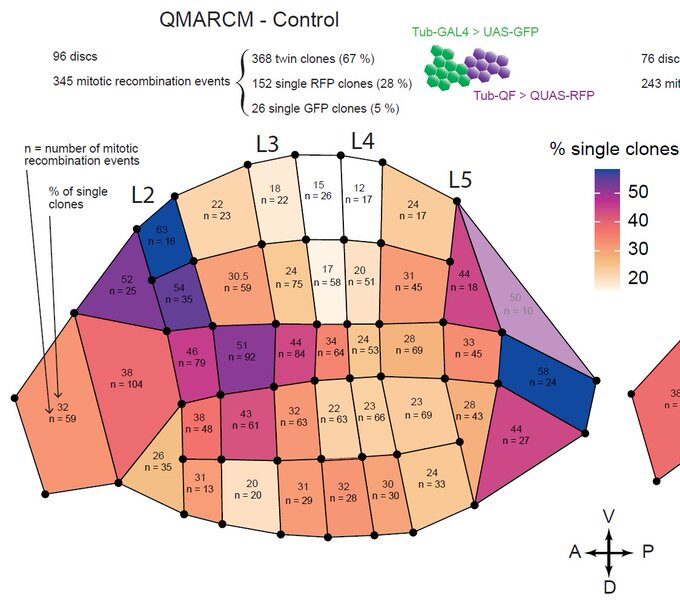
Now, using hundreds of discs and the same spatial landmark mentioned above, we could construct a map of the probability of clone disappearance and obtained this:
Basically, clones are more likely to disappear in the “hotspots” of apoptosis. Not only do clone disappear more often there, but those that remain are smaller on average, suggesting that this spatial bias of apoptosis reduces growth locally.
Could these spatial differences have an impact on the final wing size and shape?
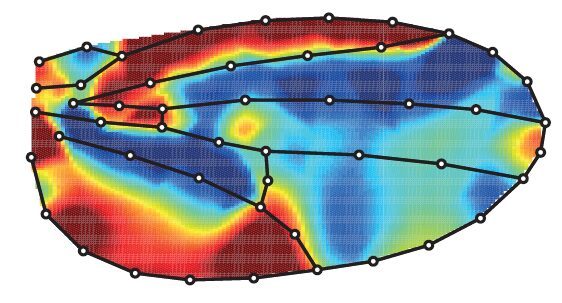
Using precise tools to automatically analyse the shape and size of hundreds of wings, we found that blocking cell death in the developing wing increases its size (by more than 8%) but also changes its shape (it is on average rounder). Below is the averaged comparison of the wing shape between the “normal” wing and the one when cell death has been blocked (red aeras are larger while blue aeras are smaller).
This was not telling us when was happening the relevant cell death. Indeed, while massive growth takes place during the larval stage, wings also undergo profound remodeling during metamorphosis at the so-called pupal stage. Using genetic tools allowing the temporal control of cell death inhibition, we were able to separate the contribution of cell death during larval and pupal stages. Doing so, we confirmed that cell death in the larvae has a strong contribution to the regulation of adult wing size, however, it also outlined a contribution of pupal apoptosis to adult wing shape.
The pupal stage offers interesting advantages as development can be easily observed “live” under the microscope. Using a “live” sensor of the regulator of cell death (the caspases, here in green) we were able to observe the dynamics of cell elimination in the pupal wing as it undergoes profound shape remodeling.
Again, this revealed that the number of cell death events was much higher than expected, with clear regions of high incidence that actually matches the domains already present in the larvae.
Overall, this work outlined the contribution of the fine tuning of spatial localisation of cell death that can be used to modulate the final shape and size of an organ/tissue (even in the phase of rapid growth). The methodology could be applied to many other systems and may reveal unexpected contributions of cell death to the regulation of shape and size of other organs.
So, to come back to our initial question, why would then a rapidly growing organ use cell elimination to modulate its shape and size (as this seems a fairly inefficient way to proceed)? Although we don’t have a firm answer to this, we believe this might help the system to be more robust to perturbations, for instance by eliminating cells that could be slightly suboptimal and affect the proper functioning of the tissue. Future work will help to assess how cell death can not only be used to modulate tissue shape, but also to provide buffering mechanism to build organ with reproducible shape and functions.
Further details can be found in the following reference:
Patterned apoptosis has an instructive role for local growth and tissue shape regulation in a fast-growing epithelium, Current Biology, January 11, 2024