🚨 Fully funded Postdoctoral and PhD fellowships available! Please contact us to apply!
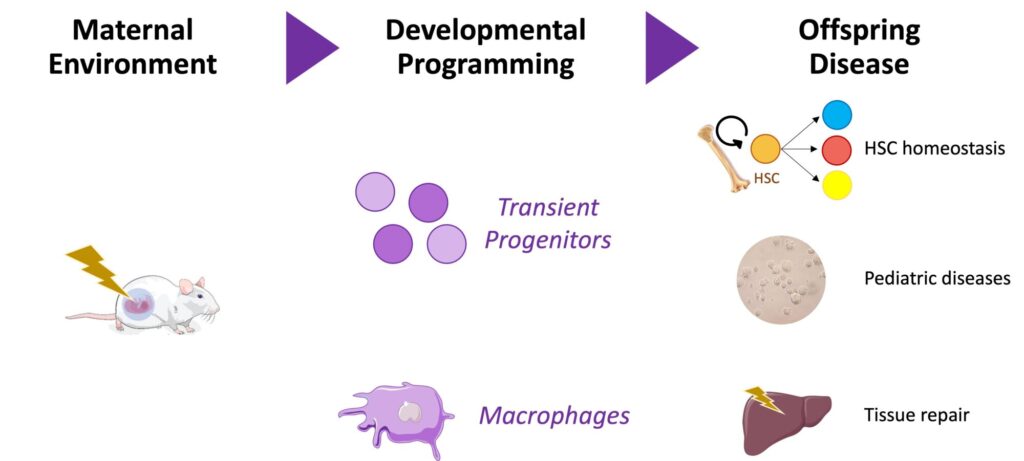
The immune system is built in successive waves that differ in cellular origin and fate, including two major embryonic waves of unclear function, temporal impact, and medical relevance. Our work has significantly contributed to the emerging notion that embryonic progenitors other than hematopoietic stem cells are responsible for blood and immune cell production during development. We will now establish how these transient embryonic progenitors and their progeny respond to prenatal challenges, convey persistent information into adulthood, and influence the risk of developing diseases after birth.
To address these questions, we will combine our expertise in fate mapping models, single cell technologies and tissue repair models to uncover how different hematopoietic progenitor waves shape the immune system from embryo to adult.
We will provide a comprehensive characterization of hematopoietic waves and determine how they cooperate within the liver hematopoietic niche to orchestrate the establishment of the immune system.
We will also investigate how genetic mutations in transient progenitors lead to pediatric blood disorders and dissect the dysregulated pathways driving disease.
Finally, we will elucidate how prenatal perturbations such as maternal inflammation affect embryonic progenitors and their long-lived progeny, the tissue resident macrophages. We will characterize the long-lasting consequences of prenatal inflammation on hematopoietic stem cell homeostasis and on macrophage functions during tissue repair.
By focusing on embryonic hematopoietic progenitors and the long-lived tissue resident macrophages they generate, we will construct a new developmental framework to explain how the immune system of the embryo contributes to the ontogeny of health and disease.
This project was selected for funding by the ERC-CoG-2024 call and the Impulscience program from fondation Bettencourt Schueller.