Lien vers Pubmed [PMID] – 10963665
Proc Natl Acad Sci U S A 2000 Aug; 97(18): 10008-13
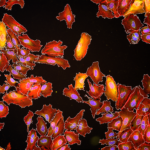
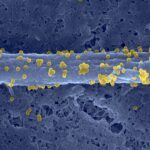
Interaction of internalin with E-cadherin promotes entry of Listeria monocytogenes into human epithelial cells. This process requires actin cytoskeleton rearrangements. Here we show, by using a series of stably transfected cell lines expressing E-cadherin variants, that the ectodomain of E-cadherin is sufficient for bacterial adherence and that the intracytoplasmic domain is required for entry. The critical cytoplasmic region was further mapped to the beta-catenin binding domain. Because beta-catenin is known to interact with alpha-catenin, which binds to actin, we generated a fusion molecule consisting of the ectodomain of E-cadherin and the actin binding site of alpha-catenin. Cells expressing this chimera were as permissive as E-cadherin-expressing cells. In agreement with these data, alpha- and beta-catenins as well as E-cadherin clustered and colocalized at the entry site, where F-actin then accumulated. Taken together, these results reveal that E-cadherin, via beta- and alpha-catenins, can trigger dynamic events of actin polymerization and membrane extensions culminating in bacterial uptake.