Link to Pubmed [PMID] – 22778100
Infect. Immun. 2012 Sep;80(9):3297-306
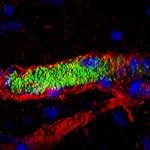
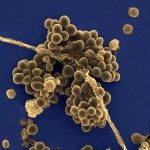
Neisseria meningitidis crosses the blood-brain barrier (BBB) following the activation of the β2-adrenergic receptor by the type IV pili (TFP). Two components of the type IV pili recruit the β2-adrenergic receptor, the major pilin PilE and the minor pilin PilV. Here, we report that a strain deleted of PilX, one of the three minor pilins, is defective in endothelial cell signaling. The signaling role of PilX was abolished when pili were not retractable. Purified PilX was unable to recruit the β2-adrenergic receptor, thus suggesting that PilX was playing an indirect role in endothelial cell signaling. Considering the recent finding that type IV pili can transition into a new conformation (N. Biais, D. L. Higashi, J. Brujic, M. So, and M. P. Sheetz, Proc. Natl. Acad. Sci. U. S. A. 107:11358-11363, 2010), we hypothesized that PilX was responsible for a structural modification of the fiber and allowed hidden epitopes to be exposed. To confirm this hypothesis, we showed that a monoclonal antibody which recognizes a linear epitope of PilE bound fibers only when bacteria adhered to endothelial cells. On the other hand, this effect was not observed in PilX-deleted pili. A deletion of a region of PilX exposed on the surface of the fiber had phenotypical consequences identical to those of a PilX deletion. These data support a model in which surface-exposed motifs of PilX use forces generated by pilus retraction to promote conformational changes required for TFP-mediated signaling.