Link to Pubmed [PMID] – 21685548
Link to DOI – 10.3851/IMP1804
Antivir Ther 2011 ; 16(4): 597-603
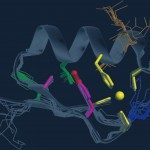
During the 2007-2008 season, A(H1N1) viruses naturally resistant to oseltamivir due to an H275Y substitution in the neuraminidase emerged and spread in the human population. The neuraminidase of 2007-2008 A(H1N1) viruses has an increased affinity for sialic acids as compared with the N1 of previously circulating viruses.Using site-directed mutagenesis analysis and an enzymatic assay on cells transiently expressing the viral neuraminidase, the amino acid changes that could account for the particular enzymatic properties of the neuraminidase of 2007-2008 A(H1N1) viruses were explored. The affinity for the substrate (K(m)) and the inhibition constants for inhibitors (K(i)) were determined for wild-type and mutated neuraminidases. Reverse genetics was used to produce 6:2 reassortant viruses expressing haemagglutinin and neuraminidase derived from A(H1N1) viruses of the 2007-2008 season or from a previously circulating H1N1 virus, in an A/WSN/33 background.The D344N substitution characteristic of the N1 of 2007-2008 A(H1N1) viruses was identified as a major determinant of its increased affinity for sialic acids. According to the viral plaque phenotype of the 6:2 reassortant viruses, the H275Y mutation was deleterious when the surface glycoproteins were derived from the H1N1 virus isolated in 2004, but not when they were derived from A(H1N1) viruses of the 2007-2008 season.The D344N substitution, by modifying the enzymatic property of the N1, may have favoured the emergence and spread of viruses naturally resistant to oseltamivir.