Link to Pubmed [PMID] – 27783975
Link to DOI – 10.1016/j.ejmech.2016.10.033S0223-5234(16)30895-9
Eur J Med Chem 2016 Nov; 124(): 1041-1056
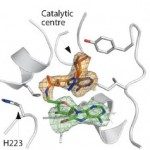
Increased resistance of pathogens to existing antibiotics necessitates the search for novel targets to develop potent antimicrobials. Biosynthetic pathways of several cofactors important for bacterial growth, such as nicotinamide adenine dinucleotide phosphate (NADP), have been proposed as a promising source of antibiotic targets. Nicotinamide adenine dinucleotide kinases (NADK; EC 2.7.1.23) are attractive for inhibitor development, since they catalyze the phosphorylation of NAD to NADP, which is an essential step of NADP metabolism. We previously synthesized diadenosine derivatives that inhibited NADK from two human pathogens, Listeria monocytogenes and Staphylococcus aureus, in the micromolar range. They behave as NAD mimics with the 5′,5′-diphosphate group substituted by a 8,5′ thioglycolic bridge. In an attempt to improve inhibitory potency, we designed new NAD mimics based on a single adenosine moiety harboring a larger derivatization attached to the C8 position and a small group at the 5′ position. Here we report the synthesis of a series of 8-thioalkyl-adenosine derivatives containing various aryl and heteroaryl moieties and their evaluation as inhibitors of L. monocytogenes NADK1, S. aureus NADK and their human counterpart. Novel, sub-micromolar inhibitors of LmNADK1 were identified. Surprisingly, most LmNADK1 inhibitors demonstrated a high selectivity index against the close staphylococcal ortholog and the human NADK. Structural characterization of enzyme-inhibitor complexes revealed the original binding mode of these novel NAD mimics.