About
The brain faces many environmental challenges throughout life. Maternal malnutrition impacts on the development of the fetal brain, and in adulthood infections such as bacterial meningitis can lead to long-term cognitive deficit. But the brain can also take advantage of environmental demands. For example exercise correlates with increased learning. Cell populations within the brain can sense and adapt to these environmental changes. Neural stem cells and their surrounding niches in particular have been shown to change their behaviour, modifying their proliferation pattern or switching to a different secretome. How they collectively react to a specific environmental challenge is an outstanding question.
Which cell populations?
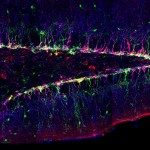
Neural stem cells are multipotent progenitors that are able to self-renew while producing neuronal and glial progeny. They are in charge of neurogenesis throughout life. They are actively proliferating during embryogenesis to build the nervous system. During adulthood they are found in a quiescent, mitotically dormant state, from which they can awake to produce new neurons. As illustration, stroke has been shown to trigger localised neurogenesis. Neural stem cells are found within a specific cellular microenvironnement, including glial cells (astrocytes), resident immune cells (microglia) and blood vessels. The vasculature provides a clear interface between the environment and the brain. Blood-borne, systemic, exogenous factors will first have to cross the physical barrier provided by the tight junctions of the endothelial cells. This blood-brain barrier not only prevents passive diffusion, but can also perform endocrine or paracrine functions targeting neural stem cells in both physiological or pathological conditions. Glial cells can also modulate neurogenesis. They are closely associated with both the neural stem cells and the vasculature, and can guide newly-born neurons towards their destination. Astrocytes have been shown to locally secrete IGF-I, a metabolic factor neural stem cells respond to to generate new neurons.
Which model?
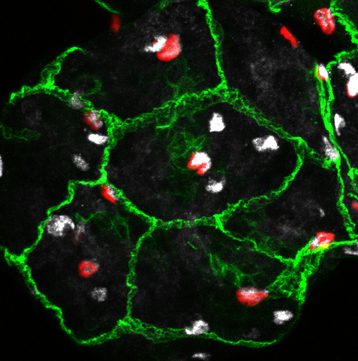
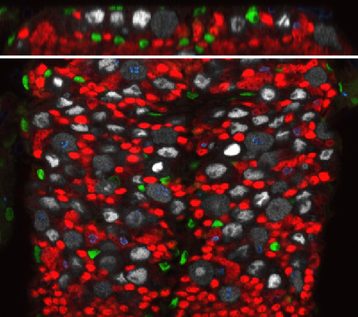
We are using a simpler yet genetically powerful model, Drosophila melanogaster, to unravel the fundamental cellular mechanisms behind brain responses to the environment. The Drosophila post-embryonic brain parallels the organisation of the vertebrate neural stem cell niche. Neural stem cells can be found either in a quiescent or proliferating state depending on the metabolic state of the organism. They are surrounded by various types of glial cells, with a wide range of functions, and by a blood-brain barrier. The Drosophila blood-brain barrier is made of glial cells, and prevents passive entrance of circulating factors by using septate junctions. It is also able to transmit metabolic signals to the stem cells. We are currently investigating the impact of environmental changes on brain function by looking at the dynamics between these three cellular populations: neural stem cells, glial niches and blood-brain barrier.
Which avenues?
We are particularly interested in the role of architectural remodelling and the dynamics of cellular interactions upon various stimuli. We want to understand i) the protective or permissive role of the blood-brain barrier towards the brain when the environmental conditions change and ii) the coordinated adaptation of glial populations to environment-induced neural stem cell behaviours. We will hugely rely on live-imaging to understand how these events unfold.