About
Research in the Innate Immunity Unit strives to understand the signals that regulate lymphoid lineage development and the mechanisms that promote the functional diversification of lymphocyte responses.
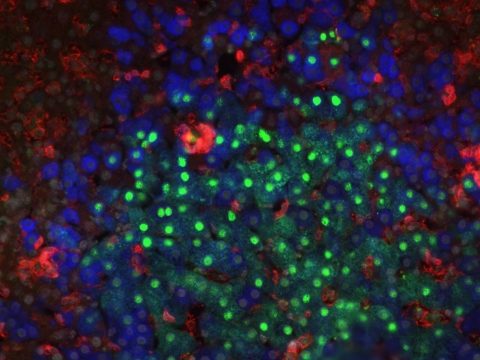
Modeling Hepatitis B virus infections in a humanized mouse model harboring a human immune system and human hepatocytes (Blue: human hepatocytes, Green: Hepatitis B virus, Red: human hematopoietic cells).
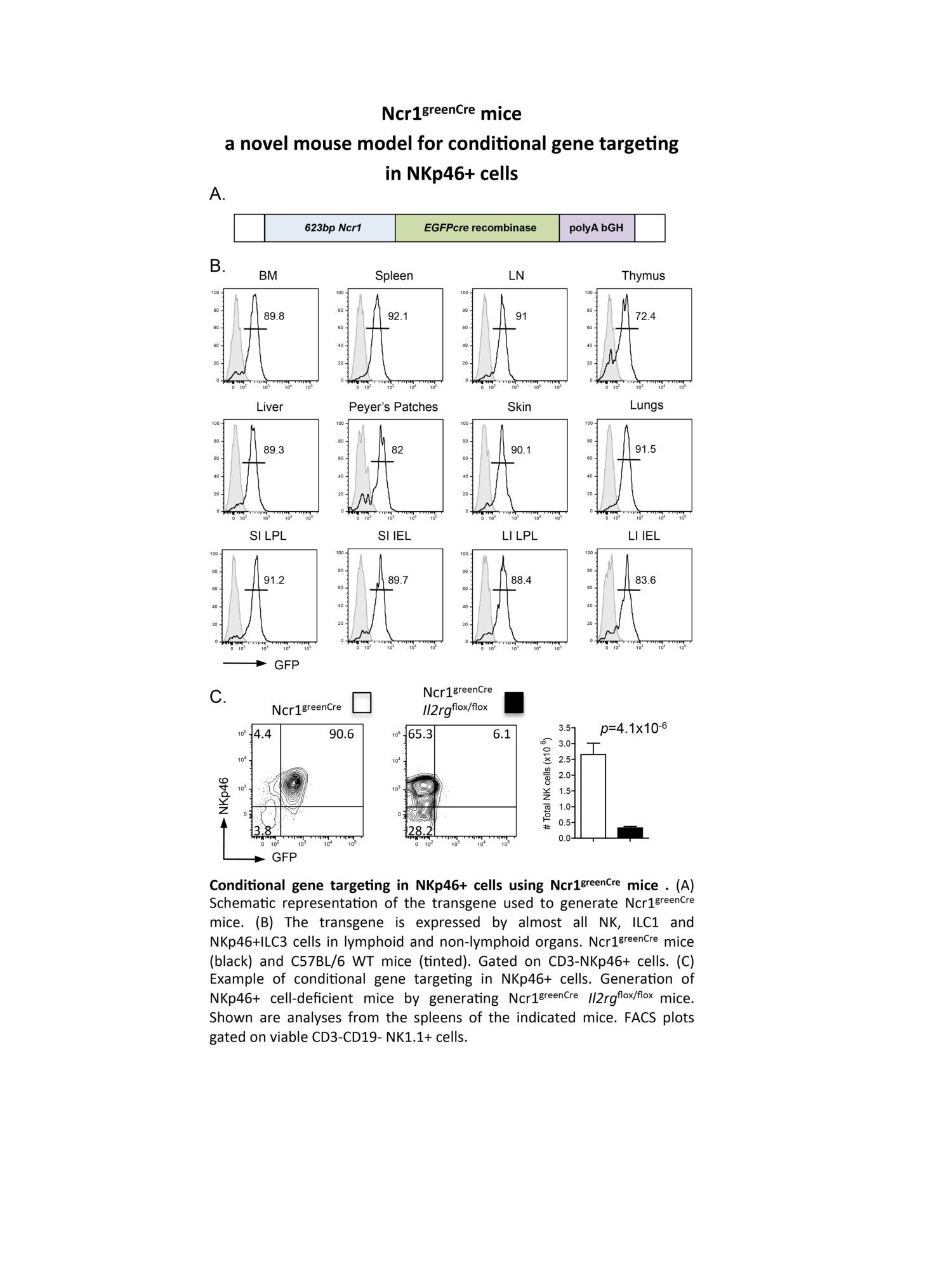
Our longstanding interest in innate lymphocytes (NK cells) has recently spread to include a new family of innate lymphoid cells (ILC) following our discovery of a novel IL-22-producing ILC in the intestine that is involved in mucosal immunity. We aim to identify the critical transcription factors that drive ILC development and to define biological roles for the different ILC subsets in different tissues.
A second theme in the laboratory involves the development of ‘humanized’ mice that harbor human cells, tissues and transgenes. Human tissue xenografts provide the means to study human immune system development and thereby provide a complementary approach to our fundamental studies of murine lymphocyte differentiation. ‘Human immune system’ (HIS) mice have been developed that can generate innate and adaptive immune responses.
Mouse models that stably harbor human hepatocyte xenografts (HuHEP mice) have also been created, allowing for a technical platform that can extend our knowledge of human liver physiology and create in vivo small animal models for human liver pathologies.These preclinical studies may provide a means to screen potential therapies for human disease. Our projects are fundamental in nature, but have important implications for future applications in clinical medicine.