Link to Pubmed [PMID] – 15897187
J. Cell. Sci. 2005 May;118(Pt 10):2201-10
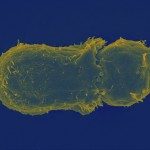
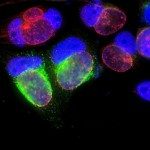
The obligate intracellular bacterium Chlamydia penetrates the host epithelial cell by inducing cytoskeleton and membrane rearrangements reminiscent of phagocytosis. Here we report that Chlamydia induces a sharp and transient activation of the endogenous small GTP-binding protein ARF6, which is required for efficient uptake. We also show that a downstream effector of ARF6, phosphatidylinositol 4-phosphate 5-kinase and its product, phosphatidylinositol 4,5-bisphosphate were instrumental for bacterial entry. By contrast, ARF6 activation of phospholipase D was not required for Chlamydia uptake. ARF6 activation was necessary for extensive actin reorganization at the invasion sites. Remarkably, these signalling players gathered with F-actin in a highly organized three-dimensional concentric calyx-like protrusion around invasive bacteria. These results indicate that ARF6, which controls membrane delivery during phagocytosis of red blood cells in macrophages, has a different role in the entry of this small bacterium, controlling cytoskeletal reorganization.