Lien vers Pubmed [PMID] – 20962348
J. Biol. Chem. 2010 Dec;285(53):41806-14
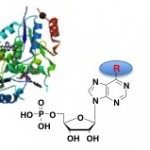
Rcl is a potential anti-angiogenic therapeutic target that hydrolyzes the N-glycosidic bond of 2′-deoxyribonucleoside 5′-monophosphate, yielding 2-deoxyribose 5-phosphate and the corresponding base. Its recently elucidated solution structure provided the first insight into the molecular basis for the substrate recognition. To facilitate the development of potent and specific inhibitors of Rcl, the active site was probed by site-directed mutagenesis and by the use of substrate analogs. The nucleobase shows weak interactions with the protein, and the deoxyribose binding pocket includes the catalytic triad Tyr-13, Asp-69, and Glu-93 and the phosphate binding site Ser-87 and Ser-117. The phosphomimetic mutation of Ser-17 to Glu prevents substrate binding and, thus, abolishes the activity of Rcl. The synthetic ligand-based analysis of the Rcl binding site shows that substitutions at positions 2 and 6 of the nucleobase as well as large heterocycles are well tolerated. The phosphate group at position 5 of the (deoxy)ribose moiety is the critical binding determinant. This study provides the roadmap for the design of small molecules inhibitors with pharmacological properties.