Lien vers HAL – hal-05085061
Lien DOI – 10.1101/2024.03.25.586565
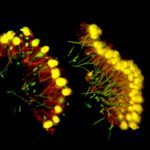
Multicellularity evolved multiple times independently during eukaryotic diversification 1-4 . Two distinct mechanisms underpin multicellularity 5 : clonal development (serial cell division of a single precursor cell) and aggregation (in which independent cells assemble into a multicellular entity). Clonal and aggregative development are traditionally considered mutually exclusive 1,6-9 , and evolutionary hypotheses have addressed why multicellular development might diverge toward one or the other extreme 3,4 . Both animals and their sister group, the choanoflagellates, are currently thought to only develop clonally 10,11 , apparently supporting an exclusively clonal pre-history for animal multicellularity 4,12 . Here, we show that the choanoflagellate Choanoeca flexa , one of the closest living relatives of animals 13 , develops into motile and contractile monolayers of cells (or “sheets”) through an unexpectedly mixed, plastic mechanism: C. flexa sheets can form by purely clonal processes, purely aggregative processes, or a combination of both. We characterize the life history of C. flexa in its natural environment, ephemeral splash pools on the island of Curaçao, and show that multicellular development is controlled by salinity during natural cycles of splash pool evaporation and refilling. Different splash pools house genetically distinct strains of C. flexa between which aggregation is constrained by kin recognition, a hallmark of aggregative multicellularity 14-17 . We propose that clonal-aggregative development allows fast and reversible transitions between unicellular and multicellular lifestyles in this rapidly fluctuating environment. Our findings challenge former generalizations about the choanoflagellate-animal lineage and expand the option space for the development and evolution of multicellularity.