Lien vers Pubmed [PMID] – 19416270
Cell. Microbiol. 2009 Aug;11(8):1179-89
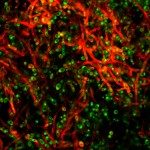
Candida albicans is a major cause of oropharyngeal, vulvovaginal and haematogenously disseminated candidiasis. Endocytosis of C. albicans hyphae by host cells is a prerequisite for tissue invasion. This internalization involves interactions between the fungal invasin Als3 and host E- or N-cadherin. Als3 shares some structural similarity with InlA, a major invasion protein of the bacterium Listeria monocytogenes. InlA mediates entry of L. monocytogenes into host cells through binding to E-cadherin. A role in internalization, for a non-classical stimulation of the clathrin-dependent endocytosis machinery, was recently highlighted. Based on the similarities between the C. albicans and L. monocytogenes invasion proteins, we studied the role of clathrin in the internalization of C. albicans. Using live-cell imaging and indirect immunofluorescence of epithelial cells infected with C. albicans, we observed that host E-cadherin, clathrin, dynamin and cortactin accumulated at sites of C. albicans internalization. Similarly, in endothelial cells, host N-cadherin, clathrin and cortactin accumulated at sites of fungal endocytosis. Furthermore, clathrin, dynamin or cortactin depletion strongly inhibited C. albicans internalization by epithelial cells. Finally, beads coated with Als3 were internalized in a clathrin-dependent manner. These data indicate that C. albicans, like L. monocytogenes, hijacks the clathrin-dependent endocytic machinery to invade host cells.