Microcalorimetry enables the thermodynamic characterization of molecular interactions in solution, and of biochemical reactions in general.
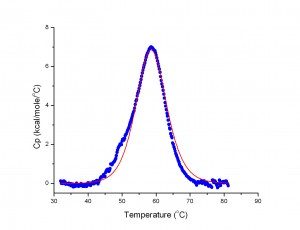
The Capillary Differential scanning calorimeter (CAP-DSC, Malvern) is principally dedicated to the study of the thermal stability of macromolecules and their complexes. By measuring the variation of heat capacity (ΔCp) as a function of temperature, DSC allows the direct determination of the variations of enthalpy (ΔH), entropy (ΔS), and the mid-transition temperature (Tm), associated to each structural transition.