Microcalorimetry enables the thermodynamic characterization of molecular interactions in solution, and of biochemical reactions in general.
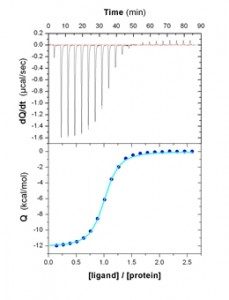
Isothermal titration calorimeters (VP-ITC et ITC200) are principally dedicated to the study of molecular interactions. By measuring the reaction heat at constant temperature, ITC allows the direct determination of binding stoichiometry (n), equilibrium constant (Ka), and variations of enthalpy (ΔH) and entropy (ΔS). Analyses at several temperatures give access to the variation of heat capacity (ΔCp) linked to the formation of a molecular complex.