Link to Pubmed [PMID] – 25376936
Chemistry 2015 Jan;21(3):1029-35
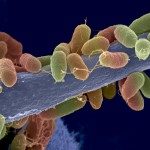
3-Aminopropyl α-(1→3)-pentaglucoside, a fragment of α-(1→3)-glucan of the cell wall of Aspergillus fumigatus, has been synthesized in a blockwise approach. The application of mono- and disaccharide N-phenyltrifluoroacetimidates bearing a stereodirecting 6-O-benzoyl group was essential for stereoselective α-glucosylations. In the products, p-methoxyphenyl and levulinoyl groups served as orthogonal protecting groups for the anomeric position and 3-OH group, respectively. Their removal from shared blocks led to donors and acceptors that were used for the synthesis of pentasaccharides. Coupling of free α-(1→3)-pentaglucoside with biotin and bovine serum albumin (BSA) gave glycoconjugate tools for mycological studies. Immunization of mice with the BSA conjugate induced the generation of antibodies that recognize α-(1→3)-glucan on A. fumigatus cell wall and distinguish its morphotypes. This discovery represents a first step to the development of a diagnostic test system and a vaccine to detect and fight this life-threatening pathogen.