Link to Pubmed [PMID] – 25168238
Cell Death Differ. 2015 Jan;22(1):108-17
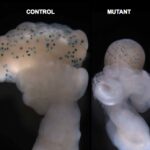
Aberrant loss of oocytes following cancer treatments or genetic mutations leads to premature ovarian insufficiency (POI) associated with endocrine-related disorders in 1% of women. Therefore, understanding the mechanisms governing oocyte death is crucial for the preservation of female fertility. Here, we report the striking reproductive features of a novel mouse model of POI obtained through oocyte-specific inactivation (ocKO) of Omcg1/Zfp830 encoding a nuclear zinc finger protein involved in pre-mRNA processing. Genetic ablation of OMCG1 in early growing oocytes leads to reduced transcription, accumulation of DNA double-strand breaks and subsequent c-Abl/TAp63-dependent oocyte death, thus uncovering the key role of OMCG1 for oocyte genomic integrity. All adult Omcg1(ocKO) females displayed complete elimination of early growing oocytes and sterility. Unexpectedly, mutant females exhibited a normal onset of puberty and sexual receptivity. Detailed studies of Omcg1(ocKO) ovaries revealed that the ovarian somatic compartment underwent a dramatic structural and functional remodeling. This allowed the cooperation between oocyte-depleted follicles and interstitial tissue to produce estradiol. Moreover, despite early folliculogenesis arrest, mutant mice exhibited sexual cyclicity as shown by cyclical changes in estrogen secretion, vaginal epithelium cytology and genital tract weight. Collectively, our findings demonstrate the key role of Omcg1 for oocyte survival and highlight the contribution of p63 pathway in damaged oocyte elimination in adulthood. Moreover, our findings challenge the prevailing view that sexual cyclicity is tightly dependent upon the pace of folliculogenesis and luteal differentiation.