About
Molecular and cellular determinants of functional synaptic diversity
At canonical fast synapses nanoscale alterations in the number, density and location of synaptic vesicles, calcium channels, receptors and fusion proteins play a critical role in defining synaptic strength and plasticity. We hypothesize that variations in these mechanisms contribute to the diversity of synaptic function observed throughout the brain. We have developed optical strategies for characterization of presynaptic function in boutons with a level of detail approaching that of classical preparation the calyx of Held. We can estimate the properties of presynaptic APs, the properties of the Ca2+ entry (duration, open probability and number of channels), and the Ca2+ channel to synaptic vesicle coupling distance. When combined with newly developed Monte Carlo-based reaction-diffusion simulations we can explore the influence of measured properties on neurotransmitter release. Here we propose to explore the molecular, cellular and biophysical properties of different synapse types to identify common motifs used to elicit different synaptic strengths and short-term plasticity profiles.
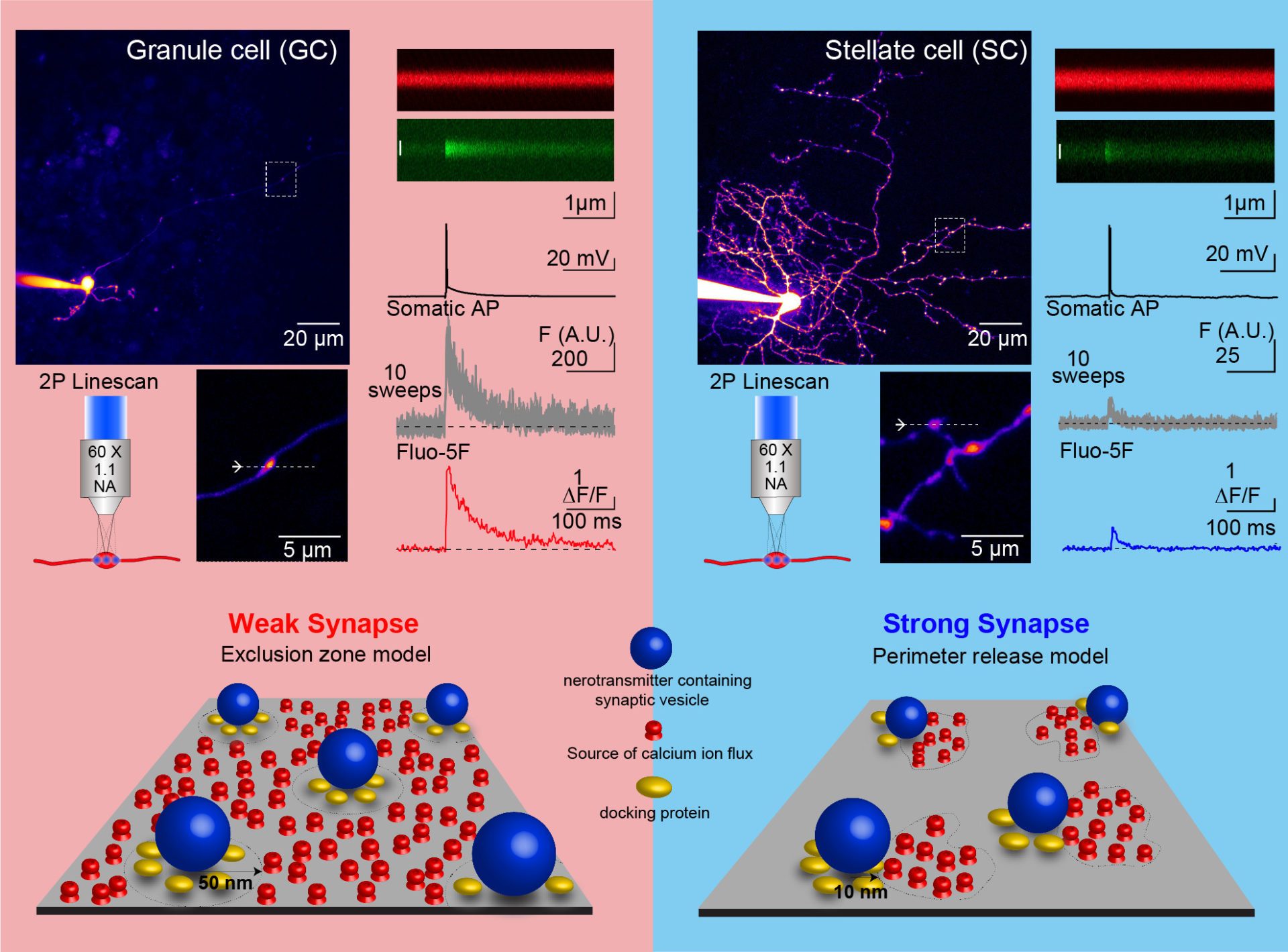
Summary: Detailed ultrastructure and biophysical experiments allowed us to propose a novel nanoscale model of synaptic transmission, which is one of the few to robustly predict the time course and strength of neurotransmitter release, and its alterations at throughout development. We therefore propose that the distance between calcium channels and synaptic vesicles is a tunable mechanism underlying the diversity of synaptic strength (Chen et al., 2015; Nakamura et al., 2015; Rebola et al. 2019, Figure 1).
Figure 1. Nanoscale diversity of synaptic protein organization influences the diversity of synaptic strength between cerebellar granule cell and stellate cell synapses (adapted from Rebola et a. Neuron, 2019)
Completed projects:
Reva, M, DiGregorio, D.A.*, and Denis S. Grebenkov, D.S.* A first-passage approach to diffusion-influenced reversible binding: insights into nanoscale signaling at the presynapse. Scientific Reports, in press.
Rebola, N., Reva, M., Kirizs, T., Szoboszlay, M., Moneron, G., Nusser, Z. and DiGregorio, D.A. Distinct nanoscale calcium channel and synaptic vesicle topographies contribute to the diversity of synaptic function. Neuron, 104(4): 693-710 (2019). Featured Article and see Preview.
Nakamura, Y., Reva, M. and DiGregorio D.A. Variations in Ca2+ influx can alter chelator-based estimates of Ca2+ channel-synaptic vesicle coupling distance. Journal of Neuroscience 8(16):3971–3987 (2018)
Chen, Z., Das, B., Nakamura, Y., DiGregorio, D.A., and Young, S.M., Jr. (2015). Ca2+ channel to synaptic vesicle distance accounts for the readily releasable pool kinetics at a functionally mature auditory synapse J Neurosci 35, 2083-2100.
Nakamura, Y., Harada, H., Kamasawa, N., Matsui, K., Rothman, J.S., Shigemoto, R., Silver, R.A., DiGregorio, D.A., and Takahashi, T. (2015). Nanoscale distribution of presynaptic Ca(2+) channels and its impact on vesicular release during development. Neuron 85, 145-158.