Lien vers Pubmed [PMID] – 19706704
J. Virol. 2009 Oct;83(20):10808-20
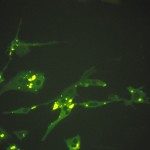
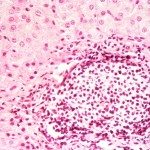
We wanted to develop a therapeutic approach against rabies disease by targeting the lyssavirus transcription/replication complex. Because this complex (nucleoprotein N-RNA template processed by the L polymerase and its cofactor, the phosphoprotein P) is similar to that of other negative-strand RNA viruses, we aimed to design broad-spectrum antiviral drugs that could be used as a complement to postexposure vaccination and immunotherapy. Recent progress in understanding the structure/function of the rabies virus P, N, and L proteins predicts that the amino-terminal end of P is an excellent target for destabilizing the replication complex because it interacts with both L (for positioning onto the N-RNA template) and N (for keeping N soluble, as needed for viral RNA encapsidation). Thus, peptides mimicking various lengths of the amino-terminal end of P have been evaluated, as follows: (i) for binding properties to the N-P-L partners by the two-hybrid method; (ii) for their capacity to inhibit the transcription/replication of a rabies virus minigenome encoding luciferase in BHK-21-T7 cells; and (iii) for their capacity to inhibit rabies virus infection of BHK-21-T7 cells and of two derivatives of the neuronal SK-N-SH cell line. Peptides P60 and P57 (the first 60 and first 57 NH2 residues of P, respectively) exhibited a rapid, strong, and long-lasting inhibitory potential on luciferase expression (>95% from 24 h to 55 h). P42 was less efficient in its inhibition level (75% for 18 to 30 h) and duration (40% after 48 h). The most promising peptides were synthesized in tandem with the Tat sequence, allowing cell penetration. Their inhibitory effects were observed on BHK-21-T7 cells infected with rabies virus and Lagos bat virus but not with vesicular stomatitis virus. In neuronal cells, a significant inhibition of both nucleocapsid inclusions and rabies virus release was observed.