Présentation
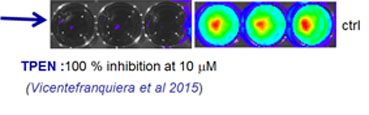
The increasing number of patients treated with cancer chemotherapy drugs in the past few decades have created an ever-expanding number of immunocompromised individuals who are at risk for opportunistic infections. As a result, opportunistic infections are at the forefront of important and often life-threatening diseases. In addition, an increasing percentage of fungi are developing resistance to available treatments. There exists a need for safer and more effective drugs to treat serious systemic fungal infections. The market for antifungal agents is a steadily growing sector driven largely by an expanding population of immunocompromised patients and the rollout of novel products. The overall aim of this project is to identify a new family of antifungal agents specifically able to inhibit the zinc metabolism of filamentous fungi. Innovative aspects rely on in vivo fungal tools allowing the high throughput screen of a very large panel of inhibitors targeting specifically the zinc transport. This project has been funded in the context of the PTR 2013 call (Programmes Transversaux de Recherche). The project is realized in collaboration with J.-P. Latgé from the Aspergillus Unit, the team of J-Antonio Calera from the Institute of functional biology in the University of Salamanca (Spain), and H Munier-Lehmann from the Unit Structural Biology and Chemistry. P. Laskaris is the Post- Doctoral fellow recruited for this project. Although numerous in vitro studies and animal models have been set up to screen the potential additive and or synergistic effects of antifungal combination therapy, with the objectives to improve the prognosis of invasive aspergillosis,to date, not only, there is not yet any widely accepted strategy but there is an urgent need to unveil the metabolic pathways of its etiological agent, A. fumigatus, that could be targeted by chemical compounds. The search for new antifungal agents targeting different metabolic pathways might be of a great interest. Identification of conventional zinc chelators able to inhibit the fungal growth altogether in vitro and in vivo and unveiling the interaction of the zinc metabolism with hypoxia Three conventional zinc chelators are used in this study (Clioquinol, Phenanthroline and TPEN). In vitro susceptibility of the A. fumigatus bioluminescent strain in liquid cultures against chelators was determined. In vitro experiments show that the most effective inhibitors among the compounds examined in this study were Clioquinol, Phenanthroline and TPEN with a MIC50 and MIC90 of 7.5 μM (example is shown in the figure below showing 100% inhibition at 10 micromolar. Luckily TPEN has also been found efficient against murine invasive aspergillosis where mice treated with 5 mg/kg/day TPEN demonstrated 60% survival in comparison with 14 % survival for the Placebo treated group. These results are very promising and experiments will be run to study in vivo interactions of using zinc chelators with conventional drugs.