Lien vers Pubmed [PMID] – 12907703
Proc. Natl. Acad. Sci. U.S.A. 2003 Aug;100(17):9962-7
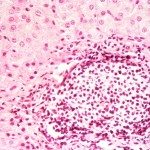
Progress in understanding the pathogenesis of hepatitis C virus (HCV) has been slowed by the absence of tractable small animal models. Whereas GB virus B (GBV-B, an unclassified flavivirus) shares a phylogenetic relationship and several biologic attributes with HCV, including hepatotropism, it is not known to cause persistent infection, a hallmark of HCV. Here, we document persistent GBV-B infection in one of two healthy tamarins (Saguinus oedipus) inoculated intrahepatically with infectious synthetic RNA. High-titer viremia (108 to 109 genome equivalents per ml) and transiently elevated serum alanine transaminase activities were present from weeks 4 to 12 postinoculation in both animals. However, whereas GBV-B was eliminated from one animal by 20 weeks, the second animal remained viremic (103 to 107 genome equivalents per ml) for >2 years, with alanine transaminase levels becoming elevated again before spontaneous resolution of the infection. A liver biopsy taken late in the course of infection demonstrated hepatitis with periportal mononuclear infiltrates, hepatocellular microvesicular changes, cytoplasmic lipid droplets, and disordered mitochondrial ultrastructure, findings remarkably similar to chronic hepatitis C. GBV-B-infected hepatocytes contained numerous small vesicular membranous structures resembling those associated with expression of HCV nonstructural proteins, and sequencing of GBV-B RNA demonstrated a rate of molecular evolution comparable to that of HCV. We conclude that GBV-B is capable of establishing persistent infections in healthy tamarins, a feature that substantially enhances its value as a model for HCV. Mitochondrial structural changes and altered lipid metabolism leading to steatosis are conserved features of the pathogenesis of chronic hepatitis caused by these genetically distinct flaviviruses.