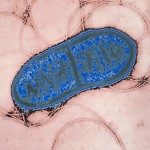
Présentation
Bacteria use front-line defenses like CRISPR-Cas and restriction-modification (R-M) systems to rapidly cleave bacteriophage DNA. However, phages have evolved mechanisms to thwart these systems including anti-CRISPR proteins, anti-RM proteins, DNA modifications, and fascinating nucleus-like structures, necessitating a co-evolutionary response for the host for the host. Here, I will describe a jumbo phage infecting the human pathogen Pseudomonas aeruginosa that avoids all tested host nucleases (CRISPR-Cas, restriction-modification, Gabija, etc.) due to two phage constructed organelles. The first is a transcriptionally active endosome-like vesicle created immediately upon infection and the second is a proteinaceous nucleus-like structure where phage DNA replication happens. These pathways ensure that the phage DNA is never exposed to the bacterial cytoplasm prior to capsid packaging. This phage family also resists signaling defense systems that use cGAS-like and TIR-like enzymes (CBASS and Thoeris, respectively) with an innovative broad spectrum inhibitor. Due to the failure of the most common defenses in P. aeruginosa, we have further identified a novel, widespread, and specific defense system “jumbo phage killer” that detects and attacks the endosome-like vesicle, preventing production of the phage nucleus and saving the cell. In summary, this phage family has innovated sophisticated and fascinating biological mechanisms due to extensive pressue from host defenses